Portal Venous Thrombosis-Disseminated Tuberculosis in Rheumatoid Arthritis
Vasanthi Natarajan1, Kevin John2, David Jose3, Pradeep Kalaiselvan4, Ashok Kumar Das5
1 Associate Professor, Department of Medicine-Endocrinology, Pondicherry Institute of Medical Sciences, Pondicherry, India.
2 Senior Resident, Department of Medicine, Pondicherry Institute of Medical Sciences, Pondicherry, India.
3 Assistant Professor, Department of Medicine, Pondicherry Institute of Medical Sciences, Pondicherry, India.
4 Senior Resident, Department of Medicine, Pondicherry Institute of Medical Sciences, Pondicherry, India.
5 Professor, Department of Medicine-Endocrinology, Pondicherry Institute of Medical Sciences, Pondicherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vasanthi Natarajan, Associate Professor, Department of Medicine, Pondicherry Institute of Medical Sciences, Kalapet, Kanagachettikulam, Pondicherry-605012, India.
E-mail: Vasanthikarthi2004@yahoo.co.in
Vascular thrombosis is one of the complications of tuberculosis. Deep vein thrombosis and pulmonary thrombosis have been reported with pulmonary and extrapulmonary tuberculosis. Splenic involvement in abdominal tuberculosis is among the rarest manifestations. Disseminated tuberculosis is predominantly reported in rheumatoid arthritis following ingestion of biological agents. Here, we report a case of disseminated tuberculosis in a rheumatoid arthritis patient who was on steroids and methotrexate for a long period, presenting with multiple splenic lesion and portal vein thrombosis, which was not reported earlier. She was treated with antitubercular drugs, anticoagulants and showed improvement.
Abdominal tuberculosis, Biological agents, Rheumatoid arthritis
Case Report
A 34-year-old female presented to medicine outpatient department with the history of cough and expectoration for 10 days duration. She had history of fever which was low grade, intermittent and also complained of loss of appetite and increased fatigability for last two months. She was diagnosed with rheumatoid arthritis five years ago by a local rheumatologist, was advised to take Tablet Prednisolone 5 mg daily and inj. Methotrexate 15 mg in every 15 days. She had stopped all the medications for last one year against medical advice.
On examination of her vitals, pulse rate was 125/min, regular rhythm, BP-100/60 mmHg, temperature 100°F, she had bilateral pitting pedal oedema. Respiratory system examination showed oxygen saturation 99% in room air, bilateral basal crepitations. Abdomen was soft with diffuse distension. Liver and spleen were enlarged. Liver was enlarged 3 cm below the right costal region, non tender. All other systemic examination was normal. Provisional diagnosis at this point was to rule out underlying tuberculosis or lymphoma.
Her blood investigation revealed total count of 4300 cells/cumm, neutrophils-67%, lymphocytes-28%, platelet 1.2 L/cumm, Hb-7.3gm% with microcytic and hypochromic picture, liver enzymes were normal. Albumin:globulin ratio was altered. Alkaline phosphatase-298 U/l, Na-122 MEq/l. Serial platelets and total count was decreased in next 72 hours upto-84000 cells/cumm, 2700 cells/cumm respectively. ECG showed sinus tachycardia, X-Ray chest was normal. Computed tomography of chest showed bilateral lower zone patchy consolidation and no evidence of interstitial lung disease. She was started on Inj. Piperacillin tazobactum 4.5 g thrice daily. Her blood and urine cultures were sterile. Sputum for Acid Fast Bacilli (AFB) was negative. Anti Neutrophilic Antibodies (ANA) by both ELISA and immunofluorescence (IF) were negative. Anti double stranded DNA, complements were normal. Virology was negative. As she had thrombocytopenia, bronchoscopy was deferred. Serum procalcitonin was 0.43 ng/dl (<1 ng/dl rules out systemic non mycobacterial infections). Ultrasonography of abdomen revealed hepatosplenomegaly with ascitis. With the presentation of fever, pancytopenia and hepatosplenomegaly differential diagnosis considered at this point were lymphoma, disseminated tuberculosis. She underwent diagnostic ascitic fluid aspiration which showed high Serum Ascitic Albumin Gradient (SAAG). Ascitic fluid Adenosine Deaminase (ADA) was 15 u/l (Normal- <80 u/l).
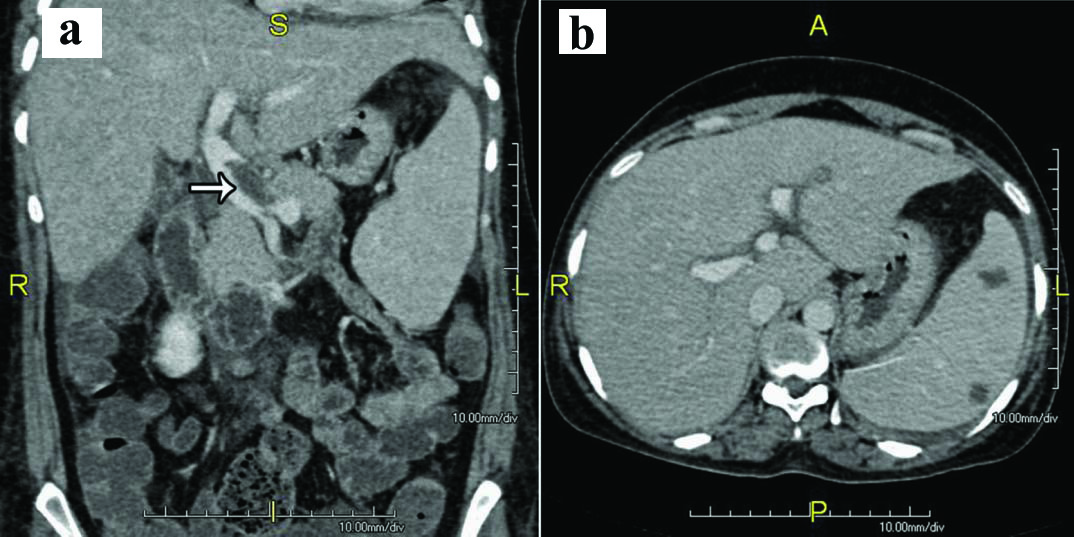
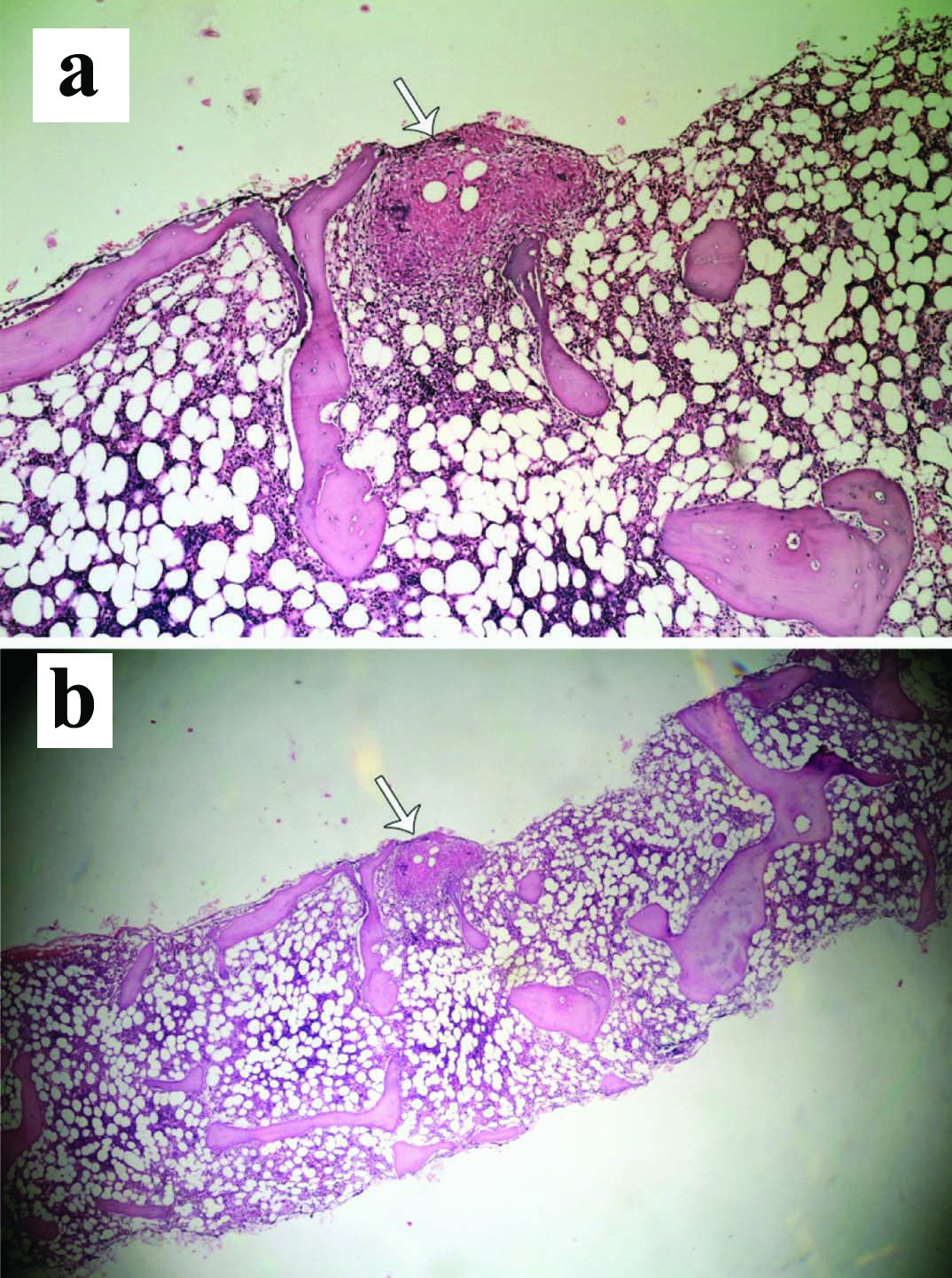
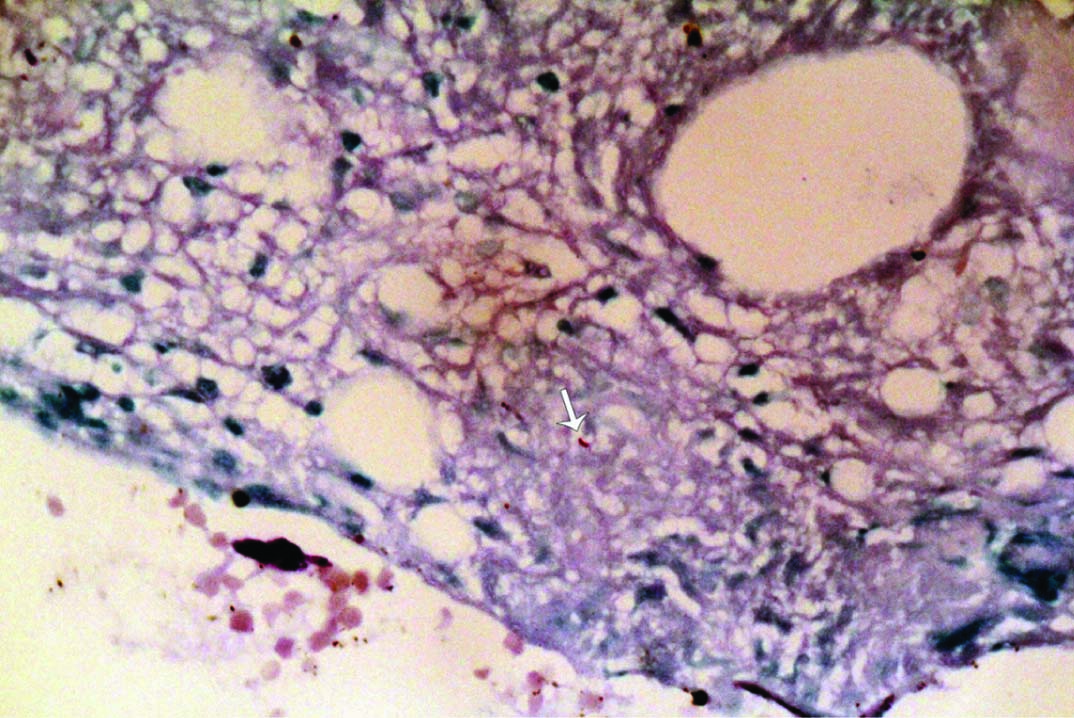
Computed tomography of abdomen with contrast showed hepatosplenomegaly with multiple hypoechoic lesions in the spleen [Table/Fig-1]. There was no abdominal lymph node enlargement. Portal vein was dilated with portal vein thrombosis. The patient underwent bone marrow aspiration and biopsy which showed caseating granulomatous lesion [Table/Fig-2a,b] and positive AFB [Table/Fig-3]. Prothrombotic evaluation including antiphospholipid antibody, c ANCA, p ANCA were negative. She was started on four antitubercular drugs (Isoniazid, Rifampicin, Pyrazinamide, Ethambutol) and oral anticoagulants (Tab. Warfarin 3 mg/4 mg alternate days). She symptomatically improved and later discharged. On follow up after three weeks of antitubercular drugs, she was having persistent joint pain, swelling which was managed with indomethazine and sulfasalazine.
Computed tomography of abdomen with contrast shows: a) Partial thrombosis in the proximal part of the main portal vein(white arrow); b) Splenomegaly with multiple hypodense splenic lesion.
Bone marrow aspiration and biopsy: a) Low power view -20X showing caseating granulomatous lesion; b) Scanner view-10X of the same lesion (white arrow).
Bone marrow aspiration- Oil immersion (100 X) with Ziehl-Neelsen stain shows positive acid fast bacilli (white arrow).
Discussion
Venous thrombosis can occur due to underlying procoagulant state, but infection related procoagulant state is under estimated. Both acute and chronic infections are prone to trigger thrombotic events. Chronic infection especially tuberculosis can produce hypercoagulable state, resulting in venous thrombosis. Many local and systemic theories explaining procoagulant nature of tuberculosis have been analyzed. In active tuberculosis underlying chronic inflammation causes thrombocytosis and increased procoagulant factors like fibrinogen, factor V111 and plasminogen activator inhibitor 1 levels and decrease in prothrombin 111 and factor C level. There is a release of pro-inflammatory cytokines and its interaction with mycobacterial pathogen releases many acute inflammatory protein causing pro-coagulant state [1,2].
Commonly reported venous thrombosis secondary to tuberculosis are deep vein thrombosis, pulmonary vein thrombosis, cortical venous thrombosis [3], splenic vein thrombosis. Both pulmonary and extra pulmonary tuberculosis [4] can cause hypercoagulable state. Disseminated tuberculosis with splenic vein thrombosis [5], tuberculous peritonitis with chronic portal venous thrombosis have also been reported [6]. Vaiphei K et al., reported multiorgan involvement of tuberculosis in a patient with venous thromboembolism involving portal and superior mesenteric vein, which was later confirmed only by autopsy [7]. Portal vein thrombosis in a patient with disseminated tuberculosis has not been reported previously, probably our case is the first case to be reported.
The occurence of venous thrombosis is more in active tuberculosis than in a person without active tuberculosis. Dentan C et al., reported that the prevalence of pulmonary embolism and venous thrombosis was 0.95% and 2.07% respectively, among the patients with active tuberculosis and 0.27% and 0.72% respectively, among the patients without active tuberculosis [8].
Tuberculosis with associated complication of venous thrombosis increases the mortality. In-hospital mortality of patients with both active tuberculosis and venous thromboembolism was 15% higher than mortality of patients with only active tuberculosis 2.7% or only venous thromboembolism 2.5% [8].
Portal venous thrombosis can occur due to underlying procoagulant state or following surgery. Among the so many causes listed, infection related especially abdominal sepsis related portal venous thrombosis is reported in 11% of cases [9]. PVT due to infective aetiology like tuberculosis is very rare. Elaine L et al., reported portal venous thrombosis and portal hypertension in a patient with isolated periportal tuberculous lymphadenitis [10]. Our patient had multiple hypodense splenic lesion. Splenic tubercular lesion occurs predominantly in a immunocompromised state. Jain D et al., reported isolated splenic vein thrombosis with splenic abscess in a young immunocompetent person [5].
Patients with inflammatory arthritis are prone for tuberculosis either pulmonary or extra pulmonary, as they are on long term immunosuppressants. In rheumatoid arthritis the occurrence of disseminated tuberculosis is predominantly reported following treatment with biological agents’ like- infiximab [11]. If it is following biological agents, patient can be managed with other drugs in subsequent days for arthritis. But in our patient it was challenging for physician to restart immunosuppressants such as steroids or methotrexate, which should be individualized with the cover of antitubercular drugs. Our patient was managed with indomethazine and sulfasalazine for arthritis.
The importance of addressing PVT is, it may extend to superior mesenteric vein and lead to bowel ischemia and gangrene. Along with ATT there is a need of anticoagulations to prevent the extension of thrombosis and bowel ischemia.
Conclusion
In active tuberculosis the risk of getting venous thrombosis as a complication is more than inactive state. Disseminated tuberculosis in a patient with rheumatoid arthritis presenting with venous thrombosis of portal vein is among the rarest presentations. Along with antitubercular drugs, anticoagulants are warranted to prevent bowel ischemia.
[1]. Hussain R, Shiratsuchi H, Phillips M, Ellner J, Wallis RS, Opsonizing antibodies (IgG1) up-regulate monocyte proinflammatory cytokines tumour necrosis factor-alpha (TNF-alpha) and IL-6 but not anti-inflammatory cytokine IL-10 in mycobacterial antigen-stimulated monocytes-implications for pathogenesisClin Exp Immunol 2001 123(2):210-18. [Google Scholar]
[2]. Kerr R, Stirling D, Ludlam CA, Interleukin 6 and haemostasisBr J Haematol 2001 115:3-12. [Google Scholar]
[3]. Vasanthi N, Sanjoy G, George E, Jeyanthi A, An unusual association: Tuberculous meningitis causing cerebral venous thrombosisJournal of Case Reports 2015 5(2):343-46. [Google Scholar]
[4]. Ruttenberg D, Graham S, Burns D, Solomon D, Bornman P, Abdominal tuberculosis: a cause of portal vein thrombosis and portal hypertensionDig Dis Sci 1991 36:112-15. [Google Scholar]
[5]. Jain D, Verma K, Jain P, Disseminated tuberculosis causing isolated splenic vein thrombosis and multiple splenic abscessesOxf Med Case Reports 2014 (6):107-09. [Google Scholar]
[6]. Wariyapperuma UM, Jayasundera CI, Peritoneal tuberculosis presenting with portal vein thrombosis and transudative Ascites - a diagnostic dilemmaBMC Infect Dis 2015 15:394 [Google Scholar]
[7]. Vaiphei K, Chawla YK, Kalra N, Multiorgan involvement in a complicated case of deep vein thrombosisIndian J Gastroenterol 2009 28(3):109-14. [Google Scholar]
[8]. Dentan C, Epaulard O, Seynaeve D, Genty C, Bosson JL, Active Tuberculosis and venous Thromboembolism: Association According to International Classification of Diseases, Ninth Revision Hospital Discharge Diagnosis CodesClin Infect Dis 2014 58(4):495-501. [Google Scholar]
[9]. Condat B, Pessione F, Hillaire S, Denninger MH, Guillin MC, Poliquin M, Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapyGastroenterology 2001 120(2):490-97. [Google Scholar]
[10]. Elaine L, Sutherland T, Slavin J, Darby J, Isolated periportal tuberculosis causing portal vein thrombosisJ Ultrasound Med 2011 30:1599-603. [Google Scholar]
[11]. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Tuberculosis associated with Infliximab, a tumor necrosis factor α–neutralizing agentN Engl J Med 2001 345(15):1098-104. [Google Scholar]