Chryseobacterium Indologenes Pneumonitis in an Infant: A Case Report
Parijat Das1, Santosh Karade2, Kanwaljit Kaur3, Ravi Ramamurthy4, Praveer Ranjan5
1 Resident, Department of Microbiology, Armed Forces Medical College, Pune, Maharashtra, India.
2 Assistant Professor, Department of Microbiology, Command Hospital (SC) and Armed Forces Medical College, Pune, Maharashtra, India.
3 Assistant Professor, Department of Microbiology, Command Hospital (SC) and Armed Forces Medical College, Pune, Maharashtra, India.
4 Assistant Professor and Pediatric Cardiologist, Department of Pediatrics, Command Hospital (SC) and Armed Forces Medical College, Pune, Maharashtra, India.
5 Professor, Department of Pathology, Command Hospital (SC) and Armed Forces Medical College, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Santosh Karade, Assistant Professor, Department of Microbiology, Command Hospital, Southern Command Pune - 411040, Maharashtra, India.
E-mail: majkarade@gmail.com
Chryseobacterium indologenes, a non-fermentative Gram-negative bacilli distributed widely in nature, is an emerging nosocomial pathogen, inherently resistant to a wide range of antibiotics. There is limited number of C. indologenes infections reported from India. We report a case of C. indologenes associated pneumonia in a three-month-old infant with congenital heart disease. This case highlights the importance of prompt diagnostic workup and targeted antibiotic therapy for its effective management.
Drug resistance, Nosocomial infection, Pneumonia, Tachypnoea
Case Report
A 10-week-old female infant suffering from complete balanced atrioventricular canal defect with severe hyperkinetic pulmonary arterial hypertension was transferred to our institute from a nearby private health care center with respiratory distress. She was being managed as a case of congestive cardiac failure with mechanical ventilation, broad spectrum antibiotics (injectable cefotaxime), anti-failure medications and supportive measures over past two weeks without much improvement. Multiple attempts to wean her off from the ventilatory support were unsuccessful. Clinical examination on arrival at our facility showed irritable child with tachycardia (146/ min), tachypnoea (36/min), raised rectal temperature (38.9°C) and oxygen saturation (SpO2) of 94%. Laboratory investigations revealed microcytic anaemia (Hb = 8.9 gm/dL), polymorphonuclear leukocytosis (WBC = 12 x 103 cells/µl with neutrophils = 76%), and raised acute-phase reactants (C-reactive protein = 20 mg/dL). Chest radiograph also showed new asymmetric bilateral pulmonary infiltrates suggestive of pneumonic consolidation in addition to features of congestive cardiac failure.
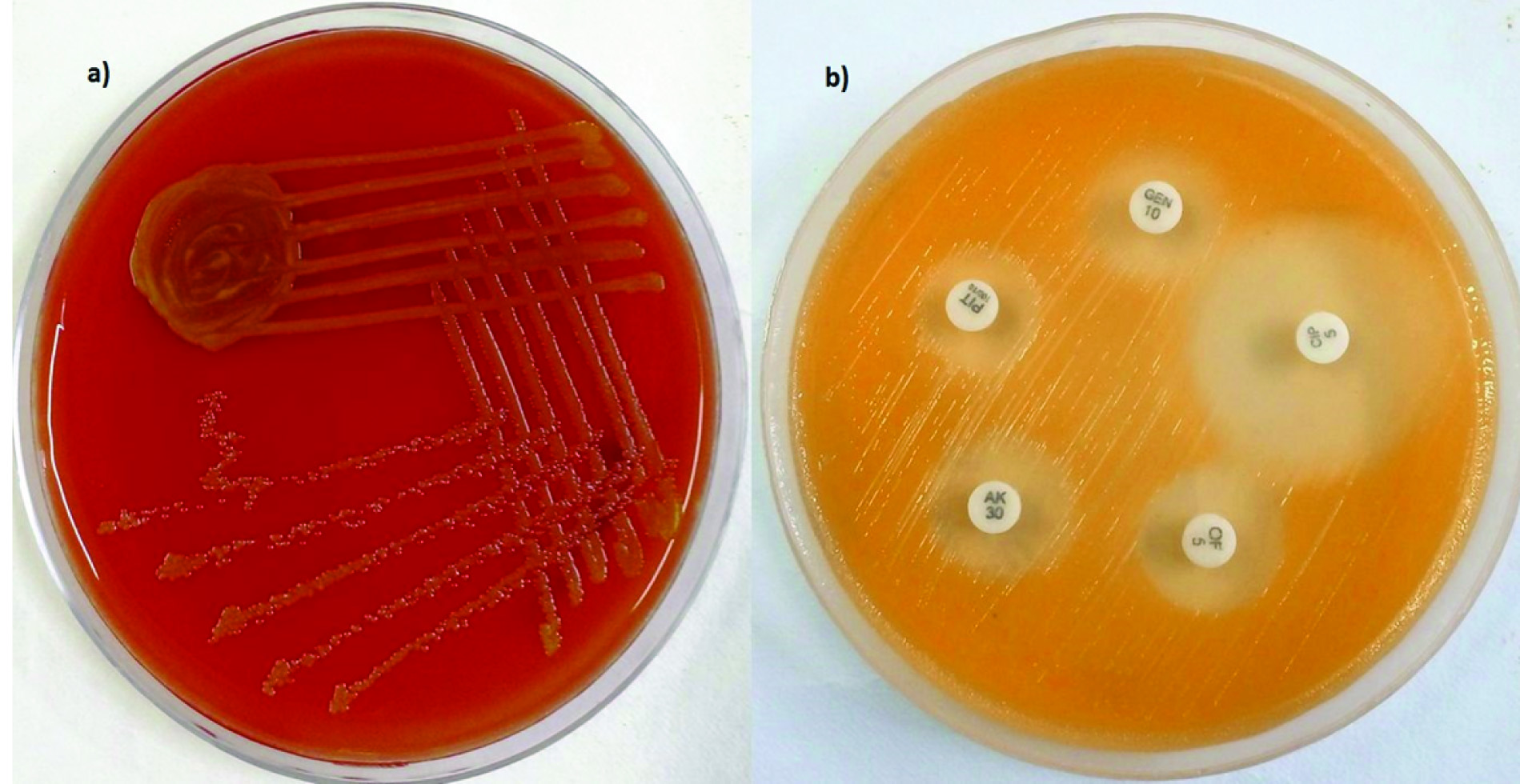
Blood, urine, Cerebrospinal Fluid (CSF) and endotracheal aspirate were sent to microbiology laboratory for bacteriological culture and she was continued on the same management. In view of ventilator dependency and refractory cardiac failure, emergency surgery for total surgical correction of the cardiac anomaly was performed on the second day of admission. During postoperative period, her ventilator and inotropic requirements were high and total leukocyte count increased further (WBC = 18.4 x 103 cells/ µl) with predominance of neutrophils (82%). Meanwhile, the Gram stained smears from thick endotracheal aspirate revealed Gram-negative bacilli with numerous polymorphonuclear leucocytes in the background. Endotracheal aspirate culture on blood agar grew yellow pigmented colonies on aerobic incubation at 37°C for 24 hours [Table/Fig-1]. These colonies were non-lactose fermenting on MacConkey and triple sugar iron agar. The organism was non-motile, positive for cytochrome oxidase activity and indole production. The isolate was identified as Chryseobacterium indologenes by VITEK 2 ID-AST (bio Merieux, France) fully automated bacterial identification system. Antibiotic susceptibility testing was also performed by VITEK 2 system and susceptibility breakpoints were interpreted based on Clinical and Laboratory Standards Institute (CLSI) recommendations for other non-Enterobacteriaceae [1]. The isolate was susceptible to ciprofloxacin, trimethoprim/sulfamethoxazole (TMP/SMX) and cefepime. Tigecycline showed intermediate susceptibility. The details of minimum inhibitory concentration for various antimicrobial agents are shown in [Table/Fig-2].
a) Yellowish orange pigmented colonies of Chryseobacterium indologenes on blood agar; b) The pigment is more obvious on Mueller Hinton agar.
Chryseobacterium indologenes minimum inhibitory concentration values for various antimicrobial agents, as determined by VITEK 2 system (bioMérieux).
| S.No | Antibiotic | MiC | interpretation |
|---|
| 1. | Ampicillin | ≥ 32 | R |
| 2. | Amoxycillin/Clavulanic acid | ≥ 32 | R |
| 3. | Piperacillin/Tazobactum | ≥ 128 | R |
| 4. | Cefuroxime | ≥ 64 | R |
| 5. | Ceftriaxone | ≥ 64 | R |
| 6. | Cefoperazone/Sulbactum | ≥ 64 | R |
| 7. | Cefepime | 8 | S |
| 8. | Colistin | ≥ 16 | R |
| 9. | Imipenem | ≥ 16 | R |
| 10. | Meropenem | ≥ 16 | R |
| 11. | Amikacin | ≥ 64 | R |
| 12. | Gentamicin | ≥ 16 | R |
| 13. | Ciprofloxacin | 1 | S |
| 14. | Trimethoprim/Suphamethoxazole | ≥ 20 | S |
| 15. | Nalidixic acid | 4 | S |
| 16. | Tigecycline | 4 | I |
Abbreviations: MIC - Minimum Inhibitory Concentration μg/ml), R - Resistant, I - Intermediate, S – Susceptible (WBC - white blood cell, hpf-high power field)
The antibiotics were changed to injectable cefepime on third postoperative day. She responded favourably with improvement of oxygen saturation and resolution of high grade fever after 72 hours of treatment. Repeat endotracheal aspirate culture performed 72 hours after initiation of antibiotic therapy was negative for Chryseobacterium. Blood, CSF and urine culture remained sterile throughout. She was weaned off from ventilatory support at the end of one week and the antibiotic therapy was continued for 14 days. Written informed consent was obtained from the parents for publication of the case and patient photographs are not used in this case report.
Discussion
Chryseobacterium indologenes has gained attention in past few years as an emerging multidrug resistance nosocomial pathogen [2]. The spectrum of infection includes meninigitis, infections of respiratory tract, urinary tract, surgical sites, soft tissue, prosthetic valve, catheter and blood stream infection [3,4]. C. indologenes belongs to the family Flavobacteriaceae. The other clinically important species of this family are C. meningosepticum and C. odoratum. These are Gram-negative, non-motile, aerobic, glucose non-fermentative, oxidase-positive bacilli. C. indologenes is easily identifiable in culture due to production of a distinct yellow to orange pigment called flexirubin [4]. There is scanty data on C. indologenes isolation from clinical specimen and its resistance profile from India [5-10]. We report a case of C. indologenes as a cause of ventilator associated pneumonia in a three-month-old infant, who was benefitted by timely institution of targeted antibiotic therapy. The Gram stained smear of endotracheal aspirate showed inflammatory cells and it was the only pathogen isolated in our case. Use of invasive medical devices, broad-spectrum antibiotics, underlying diseases and primary or acquired immunosuppressive conditions are the known risk factors for hospital acquired infection. Complete atrioventricular canal defect with congestive cardiac failure, prolonged hospital stay and mechanical ventilation were important predisposing factors in our patient.
Unless diagnosed, the choice of an appropriate antibiotic for the empirical treatment is difficult. Chen FL et al., have indicated significant 14 days survival benefit following targeted antibiotic therapy [11]. Chryseobacterium species exhibit multidrug resistance due to production of class A and class B-lactamases and are susceptible to quinolones, TMP/SMX and piperacillin– tazobactum (PIP/TAZ) combinations [2]. Our strain was resistant to PIP/TAZ, aminoglycosides, carbapenems, colistin and second/ third generation cephalosporins, which are the antibiotic of choice for empirical treatment of severe Gram-negative infections. The susceptibility to vancomycin was not tested and there is increasing trend of vancomycin resistance being reported [2,11,12]. The present isolate was susceptible to ciprofloxacin, TMP/SMX and cefepime. Injectable cefepime was initiated considering its safety and efficacy in respiratory tract infection. The patient responded favourably with improvement in oxygen saturation and general condition.
Pseudomonas, Acinetobacter and Stenotrophomonas are the commonest non-fermenters isolated in clinical laboratory. Species level identification of other non-fermenting bacilli by conventional technique is cumbersome. Isolation of a pigmented, non-motile, oxidase-positive and non-fermenting organism should raise suspicion among clinical microbiologist about possibility of C. indologenes. Newer fluoroquinolones and TMP/SMX are valuable antibiotics in such cases for empirical therapy till individualized susceptibility reports are available. Availability of automated culture and antibiotic susceptibility test techniques has improved isolation of non-fermenters. C indologenes resists chlorination and is adapted to survive in hospital environment. It can colonize medical equipments like respiratory tubing and prosthetic devices [2,11,13]. Isolation of such rare pathogen also warrants implementation of strict hospital infection control measures to limit its further spread.
Conclusion
C. indologenes is an emerging nosocomial multidrug resistance pathogen causing variety of infections among individuals of extremes of age and immunocompromised patients. Early diagnostic workup and targeted antibiotic therapy are essential for its effective management.
Abbreviations: MIC - Minimum Inhibitory Concentration μg/ml), R - Resistant, I - Intermediate, S – Susceptible (WBC - white blood cell, hpf-high power field)
[1]. Performance standards for antimicrobial susceptibility testingCLSI supplement M100S (ISBN 1-56238-923-8 [Print];ISBN 1-56238-924-6 [Electronic]) 2016 26th editionWayne, PA, USAClinical and Laboratory Standards Institute [Google Scholar]
[2]. Kirby JT, Sader HS, Walsh TR, Jones RN, Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp: report from the SENTRY Antimicrobial Surveillance Program (1997-2001)J Clin Microbiol 2004 42:445-48. [Google Scholar]
[3]. Aykac K, Ozsurekci Y, Tuncer O, Sancak B, Cengiz AB, Kara A, Six cases during 2012-2015 and literature review of Chryseobacterium indologenes infections in pediatric patientsCan J Microbiol 2016 62:812-19. [Google Scholar]
[4]. Mukerji R, Kakarala R, Smith SJ, Kusz HG, Chryseobacterium indologenes: an emerging infection in the USABMJ Case Rep 2016 :bcr2016214486 [Google Scholar]
[5]. Baruah M, Lyngdoh C, Lyngdoh WV, Talukdar R, Noncatheter-related bacteraemia due to Chryseobacterium indologenes in an immunocompetent patientIndian J Med Microbiol 2016 34:380-81. [Google Scholar]
[6]. Bhuyar G, Jain S, Shah H, Mehta VK, Urinary tract infection by ChryseobacteriumindologenesIndian J Med Microbiol 2012 30:370-72. [Google Scholar]
[7]. Shahul HA, Manu MK, Chryseobacterium indologenes pneumonia in a patient with non-Hodgkin’s lymphomaBMJ Case Rep 2014 doi: 10.1136/bcr-2014-204590 [Google Scholar]
[8]. Srinivasan G, Muthusamy S, Raveendran V, Joseph NM, Easow JM, Unforeseeable presentation of Chryseobacterium indologenes infection in a paediatric patientBMC Res Notes 2016 9:212 [Google Scholar]
[9]. Radera S, Tripathi S, Agarwal J, Kumar M, Chryseobacterium indologenes associated pneumonia in two neonatesPediatr Infect Dis J 2017 36(3):337-39. [Google Scholar]
[10]. Eshwara VK, Sasi A, Munim F, Purkayastha J, Lewis LE, Mukhopadhyay C, Neonatal meningitis and sepsis by Chryseobacterium indologenes: a rare and resistant bacteriumIndian J Pediatr 2014 81:611-13. [Google Scholar]
[11]. Chen FL, Wang GC, Teng SO, Ou TY, Yu FL, Lee WS, Clinical and epidemiological features of Chryseobacterium indologenes infections: analysis of 215 casesJ Microbiol Immunol Infect 2013 46:425-32. [Google Scholar]
[12]. Chou DW, Wu SL, Lee CT, Tai FT, Yu WL, Clinical characteristics, antimicrobial susceptibilities, and outcomes of patients with Chryseobacterium indologenes bacteremia in an intensive care unitJpn J Infect Dis 2011 64:520-24. [Google Scholar]
[13]. Nulens E, Bussels B, Bols A, Gordts B, Van Landuyt HW, Recurrent bacteremia by Chryseobacterium indologenes in an oncology patient with a totally implanted intravascular deviceClin Microbiol Infect 2001 7:391-93. [Google Scholar]