Cytology of Collagenous Spherulosis: A Case Series
Devi Subbarayan1, Vijayashree Raghavan2
1 Assistant Professor, Department of Pathology, Chettinad Health City and Research Institute, Kelambakkam, Tamil Nadu, India.
2 Professor, Department of Pathology, Chettinad Health City and Research Institute, Kelambakkam, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Devi Subbarayan, Assistant Professor, Department of Pathology, Chettinad Health City and Research Institute, D Block, Kelambakkam-603103, Tamil Nadu, India.
E-mail: sdevi2001@gmail.com
Collagenous Spherulosis (CS) is an uncommon benign entity characterized by the presence of pink hyaline globules composed of basement membrane like material. Although they are known to occur independently, they are more often found as incidental findings in association with benign pro iferative breast lesions. As morphologically similar hyaline globules also occur in certain malignant lesions such as Adenoid Cystic Carcinoma (ACC) and adenomyoepithelial carcinoma, their presence may pose diagnostic problems. The present series is a retrospective analysis of six cases of CS reported cytologically over a period of three years from 2013 to 2016 at Chettinad Hospital and Research, Tamil Nadu, India. The cytopathologist should be aware of this benign entity to distinguish it from similar looking malignant lesions to avoid inappropriate management.
Adenoid cystic carcinoma, Collagen spherule, Differential diagnosis
Clement PB and his associates first described CS on histological sections in 1987 [1]. It is recognized by the presence of eosinophilic hyaline globules rimmed by a layer of myoepithelial cells. These globules are morphologically similar to those observed in ACC and are formed from basement membrane like material. CS is considered to be a special form of ductal hyperplasia usually associated with a variety of benign proliferative breast lesions [1-3]. However, sometimes diagnostic difficulties may arise due to its close morphological resemblance to certain malignant lesions such as ACC and adenomyoepithelial carcinoma, a fact that cytopathologists should keep in mind to avoid misinterpretation [4,5].
Case Series
In this series, we present retrospective cytological analysis of six cases of CS reported in our institute over a period of four years from 2013 to 2016. Out of 984 breast FNACs performed during this period, six cases of CS were reported. FNAC was performed using 22-24 gauge needles and the dried smears were stained with Leishman stain.
The following cytological features were analysed: 1) Cellularity; 2) Presence of epithelial and myoepithelial cell component; 3) Cell arrangement; 4) Nuclear atypia; and 5) Background and other cell types including the presence of bare bipolar nuclei, apocrine change, foamy macrophages, stromal fragments, calcification and acellular spherical basement membrane material. In addition, relevant clinical details were obtained in all cases and mammographic data was obtained when it was available.
All six patients were women, aged between 23 and 62 with a mean age at presentation of 39 years. Clinical details were available in all cases but mammographic data was available in only three cases [Table/Fig-1]. Cytomorphological features are shown in [Table/Fig-2].
Clinical features and mammographic data with cytological diagnosis.
| Case no | Age | Clinical presentation | Mammographic impression | Cytological diagnosis |
|---|
| 1 | 39 | Bilateral breast, vague nodularity | BIRADS II | CS with benign proliferative breast disease |
| 2 | 62 | Right breast, RUQ | BIRADS II | CS with benign proliferative breast disease |
| 3 | 29 | Left breast, RUQ | | CS with fibrocystic disease |
| 4 | 55 | Right breast, subareolar | | CS with p/o subareolar duct papillomatosis |
| 5 | 26 | Right breast | | CS with fibrocystic disease |
| 6 | 23 | Left breast, LUQ | BIRADS III | CS with atypical ductal hyperplasia |
BIRADS - Breast Imaging - Reporting and Data System. RUQ- Right Upper Quadrant, LUQ- Left I Upper Quadrant
| Cytomor- phological features | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|
| Cellularity | ++ | ++ | ++ | Acellular [Table/Fig-3] | ++ | ++ |
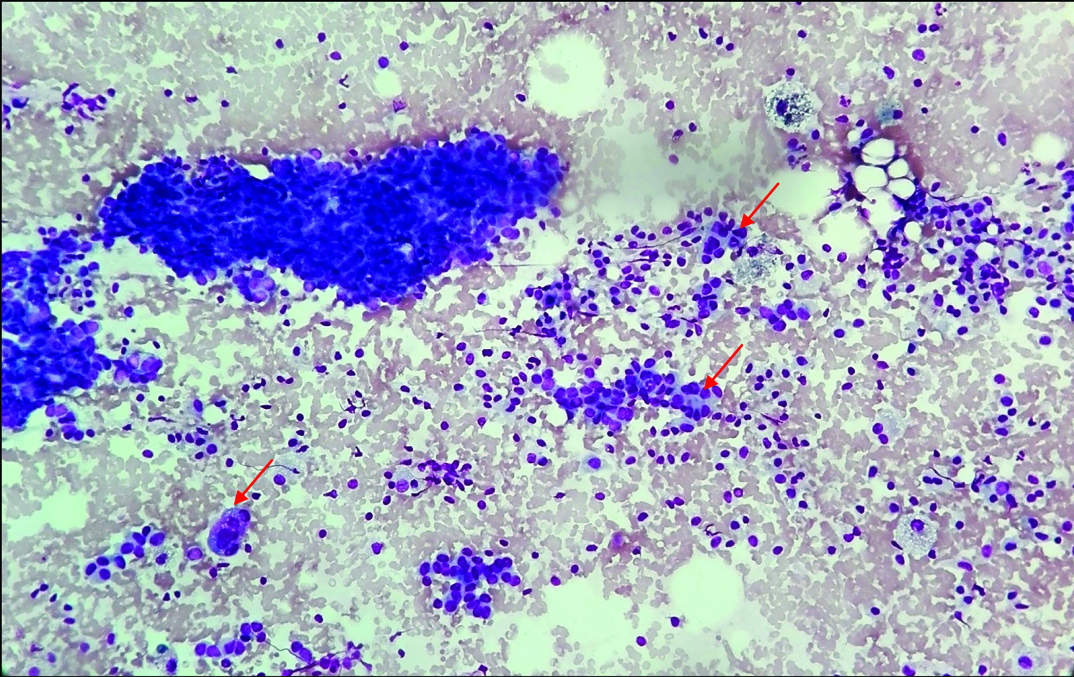
| Biphasic cell population (ductal Epithelial cells and myoepithelial cells) | ++ | ++ | ++ | - | ++ | Sparse myoepithelial cells in some clusters [Table/Fig-4] |
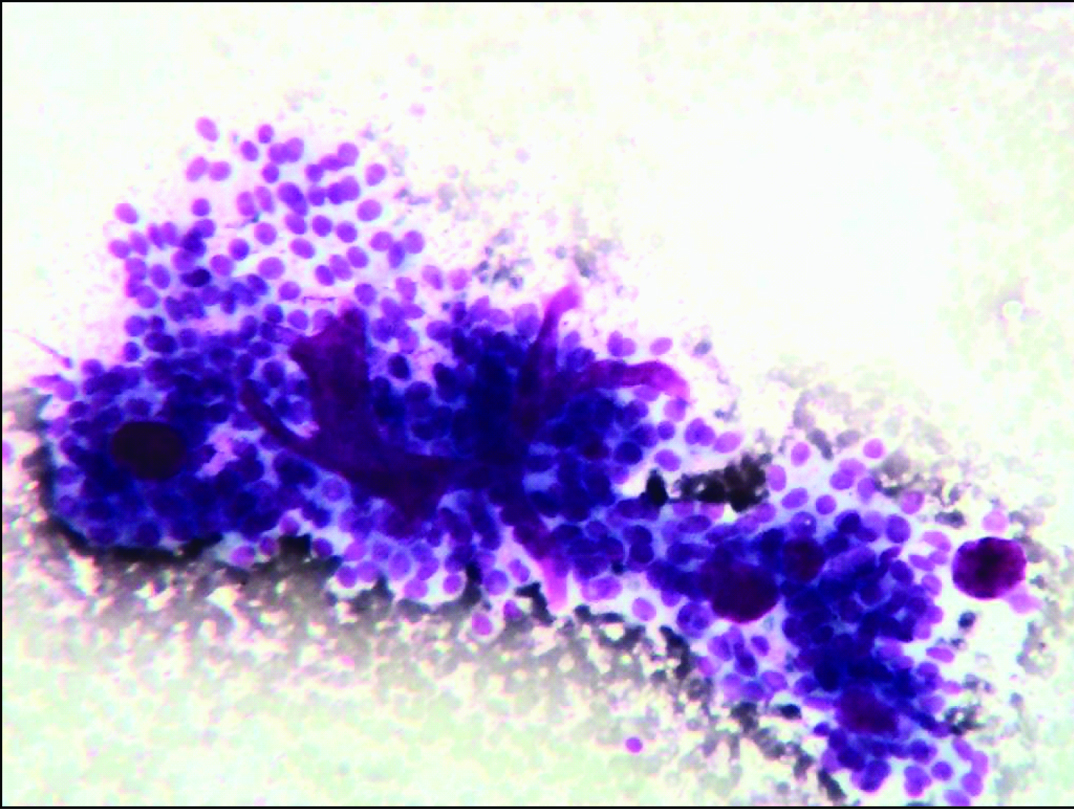
| Cell arrangement | Cohesive groups and sheets | Cohesive groups and sheets | Cohesive groups and sheets | - | Cohesive groups and sheets | Cohesive groups of ductal epithelial cells, focal cribriform pattern [Table/Fig-5] |
| Nuclear atypia | - | - | - | - | - | Mild anisonucleosis, [Table/Fig-4] nuclear overlapping |
| Bare bipolar nuclei | + | + | + | - | + | + |
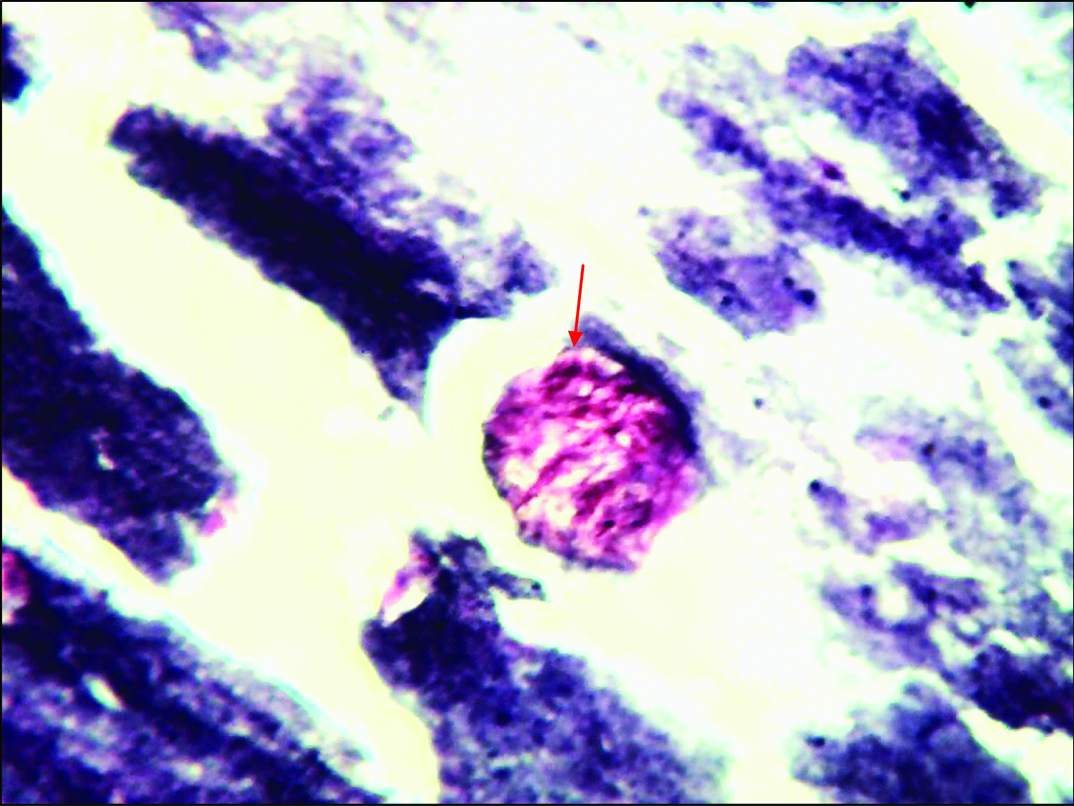
| Collagenous spherules | + [Table/Fig-6] | + [Table/Fig-7] | + [Table/Fig-8] | + [Table/Fig-3] | + [Table/Fig-9] | + |
| Other features and background apocrine change and foamy macrophages | - | - | + | - | + | + |
| Calcification | - | - | - | + [Table/Fig-3] | - | - |
++ - moderate to high, + - present. Two cases (case 4 and 6) were advised excision and histo- pathological correlation. Unfortunately the cases were lost in follow up
Acellular smear showing collagen spherule (arrow) with calcification in the background (Leishman stain, 40X).
Cellular smear showing mild anisonucleosis with sparse myoepithelial cells in some clusters. (arrow) (Leishman stain, 40X).
Cohesive group of ductal epithelial cells forming cribriform pattern (Leishman stain, 40X).
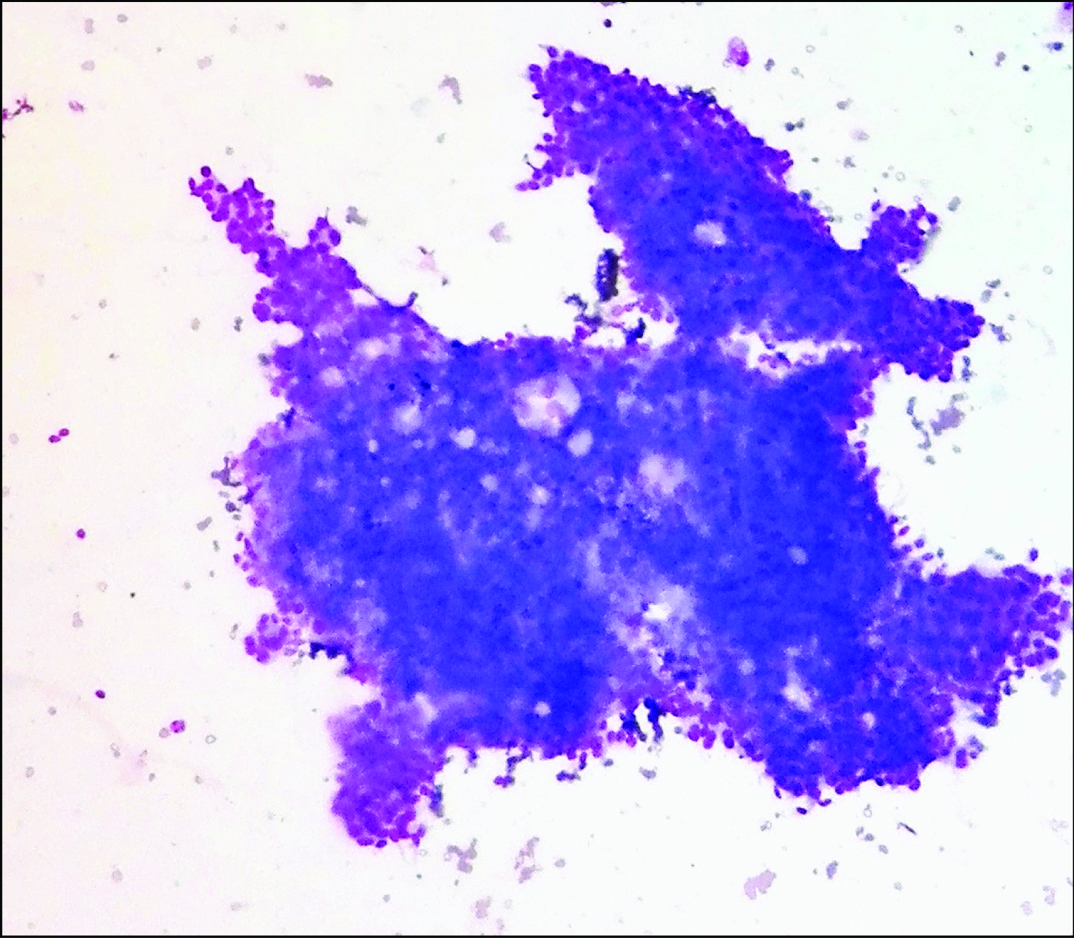
Cellular smear showing branching monolayered sheets of epithelial and myoepithelial cells intermingled with pink hyaline globules and basement membrane material (Leishman stain, 40X).
Smear showing collagen spherule with adherent myoepithelial cells (Leishman stain, 100X).
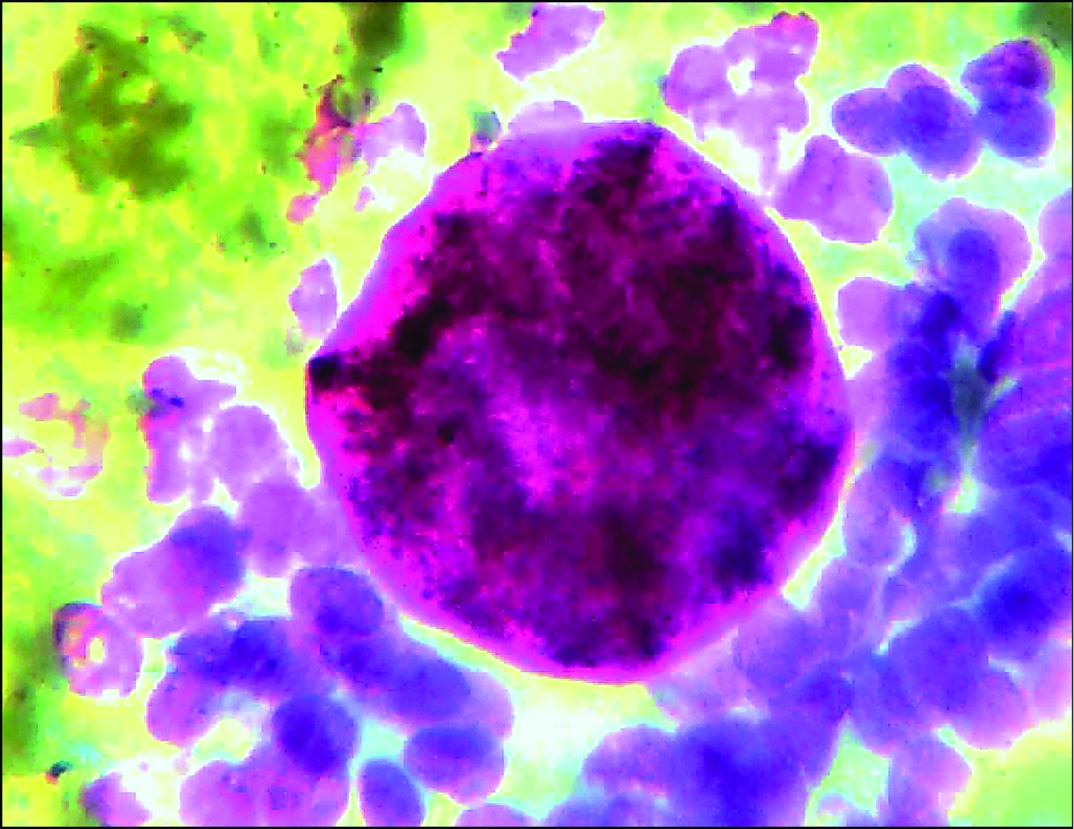
Smear showing multiple collagen spherules with branching sheets of ductal epithelial cells. (Leishman stain, 40X).
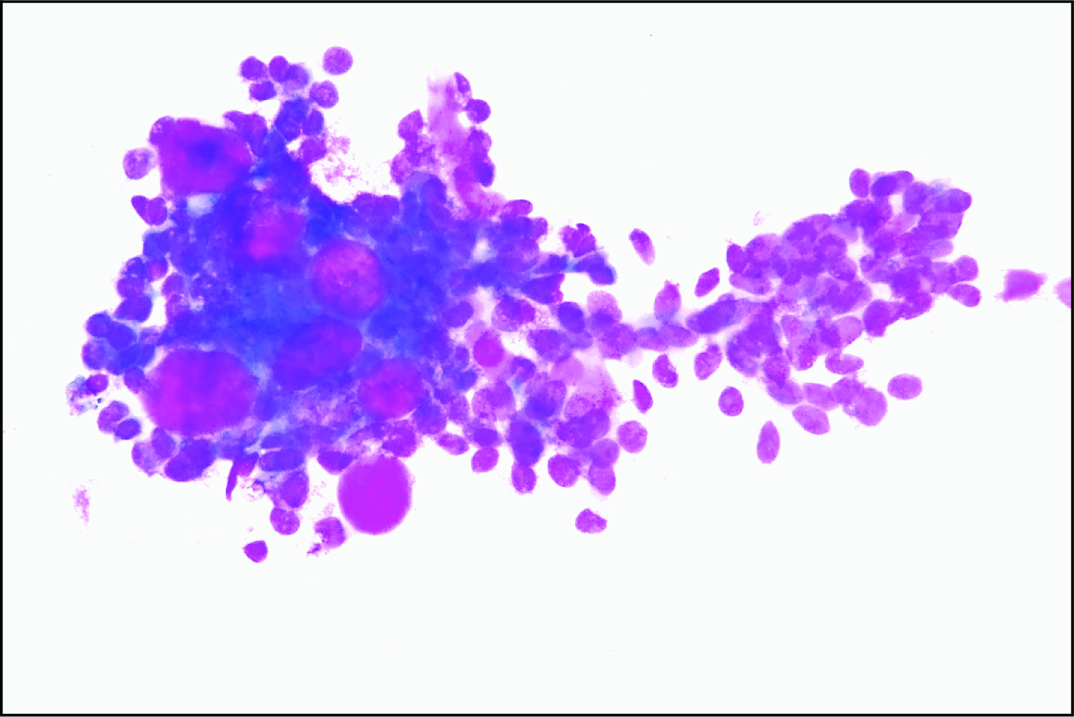
Smear showing collagen spherule with ductal epithelial cells and basement membrane material. (Leishman stain, 40X).
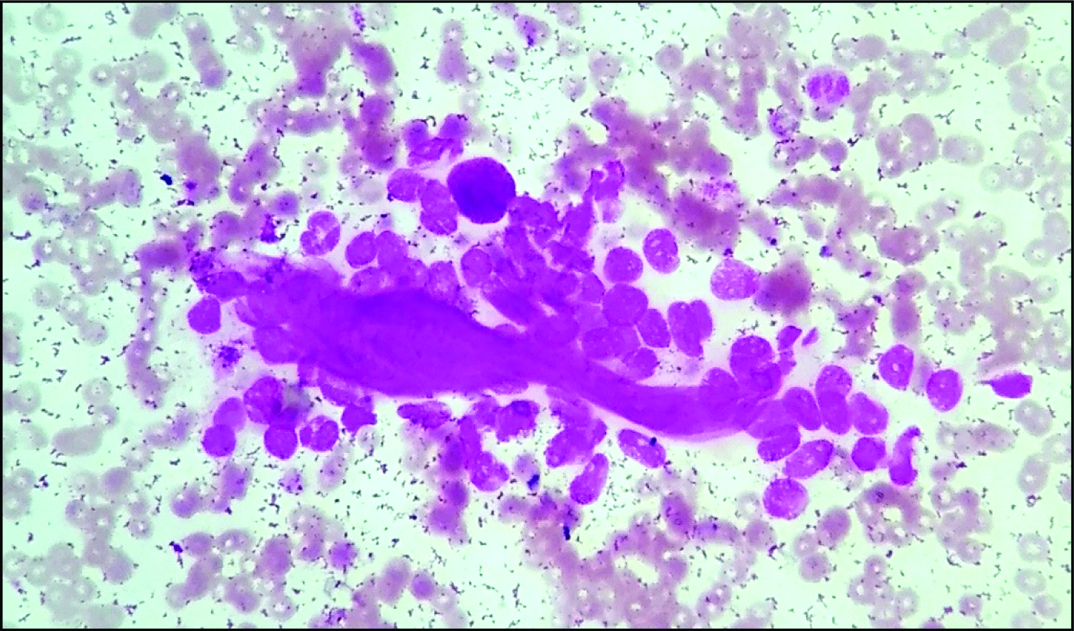
Discussion
CS is an uncommon benign entity usually seen between third and sixth decade of life. It was first described by Clement PB et al., in 1987 [1]. The incidence of CS on cytological smears is <0.2% of all breast smears, while on histological sections it is seen in <1% of biopsies from the breast [2]. CS per se is an innocuous morphological change seen in palpable as well as non-palpable breast masses, most commonly associated with hyperplastic duct lesions. Benign proliferative breast lesions such as papilloma, papillary duct hyperplasia, sclerosing adenosis, radial scar, fibrocystic disease, adenomyoepithelioma and atypical ductal hyperplasia are also associated with CS [1-3].
Histologically, acellular eosinophilic spheres of 20 to 100 µm of diameter are characteristic feature of CS [1]. Functional myoepithelial cells are known to produce basement membrane material which forms spherical hyaline masses. Cytologically these spherules appear pink and acellular on Romanowsky stained smears. Histochemical and immunohistochemical studies have shown that this material is positive for laminin and type IV collagen, implying its formation from materials normally found in basement membrane [4]. Electron microscopic studies have identified the cells surrounding these globules as of myoepithelial differentiation [4]. Though in most cases these hyaline globules consist of basement membrane like material, in few cases, the material is found to be mature collagen suggesting that the chemical composition of globules is related to the degree of myoepithelial differentiation [4]. Mooney EE et al., have described immunohistochemically similar structures, but with basophilic appearance, as mucinous spherulosis. They may represent an early stage in development of CS and, with time, may evolve into mature eosinophilic globules [5]. Degenerative changes like calcification, cystic and myxoid change can occur in CS [6]. Calcification has been described in 25% of the cases of CS [7]. One of our cases in which FNAC yielded acellular material was associated with calcification (Case 5). CS with acellular smears has not been described in the previous studies. Though cystic change in the absence of epithelial elements has been described, calcification in the absence of epithelial elements has not been described in literature so far [8].
Apart from benign proliferative lesions, myoepithelial cells also participate in rare malignant lesions such as ACC and epithelial myoepithelial carcinoma of breast wherein similar globules are produced. Cytologically, these conditions pose a diagnostic challenge and should be differentiated from CS to prevent inappropriate management. Cribriform hyperplasia sometimes forms true glands along with hyaline globules that resemble ACC. However, in CS, the spherules are surrounded by single layer of myoepithelial cells in contrast to several layers of cells seen in ACC. Other differentiating features that favour CS include presence of monolayered sheets, low N:C ratio and bare bipolar nuclei in the background. Presence of three dimensional clusters with nuclear crowding and overlapping, high N:C ratio and absence of bipolar nuclei in the background support the diagnosis of ACC [8]. In difficult cases biopsy and immunohistochemistry will solve the problem. The cells of ACC will be positive for C-kit (CD117), while in CS, it will be negative [8,9].
Conclusion
Despite its benign nature, the diagnosis of CS on cytological smears is challenging. Cytologically, it can masquerade as ACC. Presence of hyaline globules and cribriform pattern in some benign proliferative lesions superficially resemble ACC. The cytopathologist should be aware of this benign lesion to avoid misinterpretation. In difficult cases biopsy and immunohistochemistry is advised to avoid inappropriate management.
BIRADS - Breast Imaging - Reporting and Data System. RUQ- Right Upper Quadrant, LUQ- Left I Upper Quadrant
++ - moderate to high, + - present. Two cases (case 4 and 6) were advised excision and histo- pathological correlation. Unfortunately the cases were lost in follow up
[1]. Clement PB, Young RH, Azzopardi JG, Collagenous spherulosis of the breastAm J Surg Pathol 1987 11(6):411-17. [Google Scholar]
[2]. Puri S, Mohindroo S, Gulati A, Collagenous spherulosis: An interesting cytological finding in breast lesionCytojournal 2015 12:25 [Google Scholar]
[3]. Resetkova E, Albarracin C, Sneige N, Collagenous spherulosis of breast: Morphologic study of 59 cases and review of the literatureAm J Surg Pathol 2006 30(1):20-27. [Google Scholar]
[4]. Wells CA, Wells CW, Yeomans P, Vifia M, Jordan S, D’Ardenne AJ, Spherical connective tissue inclusions in epithelial hyperplasia of the breast (“collagenous spherulosis”)J Clin Pathol 1990 43:905-08. [Google Scholar]
[5]. Mooney EE, Kayani N, Tavassoli FA, Spherulosis of the breast. A spectrum of mucinous and collagenous lesionsArch Pathol Lab Med 1999 123:626-30. [Google Scholar]
[6]. Rosen PP, Rosen PP, Papilloma and related benign tumorsRosen’s breast pathology 2009 3rd edLippincott Williams & Wilkins:130-32. [Google Scholar]
[7]. Bavle RM, Collagenous spherulosisJ Oral Maxillofac Pathol 2013 17(3):322-23. [Google Scholar]
[8]. Pandya AN, Shah P, Patel RD, Patel PR, Adenoid cystic carcinoma of breast and the importance of differentiation from collagenous spherulosis by FNACJournal of Cytology 2010 27(2):69-70. [Google Scholar]
[9]. Rabban JT, Swain RS, Zaloudek CJ, Chase DR, Chen YY, Immunophenotypic overlap between adenoid cystic carcinoma and collagenous spherulosis of the breast: potential diagnostic pitfalls using myoepithelial markersMod Pathol 2006 19(10):1351-57. [Google Scholar]