Enhanced Expression of FRA16B using AT-Rich DNA Binding Chemicals in a Woman with Secondary Amenorrhoea
Gunasekaran Bhavani1, S Sivaprakash2, Chandra R Samuel3, Sathiyavedu Thyagarajan Santhiya4
1 Ph.D. Research Scholar, Department of of Genetics, Dr. ALM Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai, Tamil Nadu, India.
2 Assistant Professor, Department of Endocrinology and Diabetology, Institute of Obstetrics and Gynaecology, Government Hospital for Women and Children, Egmore, Chennai, Tamil Nadu, India.
3 Associate Professor, Department of of Genetics, Dr. ALM Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai, Tamil Nadu, India.
4 Professor and Head (Retd.), Department of of Genetics, Dr. ALM Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sathiyavedu Thyagarajan Santhiya, Professor and Head (Retd.), Department of Genetics, Dr. ALM PG IBMS, University of Madras, Taramani, Chennai, Tamil Nadu, India.
E-mail: v_santhiya63@hotmail.com
Fragile sites represent regions of chromatin that fail to compact during mitosis. Based on the prevalence and pattern of inheritance they are classified as rare fragile sites or common fragile sites. Rare fragile sites either occur spontaneously or can be induced by certain AT-specific binding chemicals namely distamycin, Hoechst 33258, Berenil and others. The most common of all rare autosomal fragile sites is fra(16)(q22) with a heterozygote frequency of ~5%. FRA16B results from an expansion of a 33 bp AT-rich Minisatellite repeat. These rare forms are usually heritable and segregate in a Mendelian fashion. The proband who was referred for secondary amenorrhoea, revealed 46,XX,fra(16)(q22.1)pat karyotype. Her father and younger sibling were also found to be carriers. This study aimed to delineate the genotypic and phenotypic features exhibited by these carriers and to evaluate FRA16B expression using AT-specific binding chemicals. The additives employed were Berenil, BrdU and Hoechst 33258. Berenil at a concentration of 150 µg/ml showed the highest expression of FRA16B. Although the recent breakthrough in molecular characterization of fragile sites plays a critical role in comprehending their association with various diseases, the physiological link between them and amenorrhoea is not clearly understood.
Amenorrhoea, Berenil, Fragile sites, Hoechst 33258
Case Report
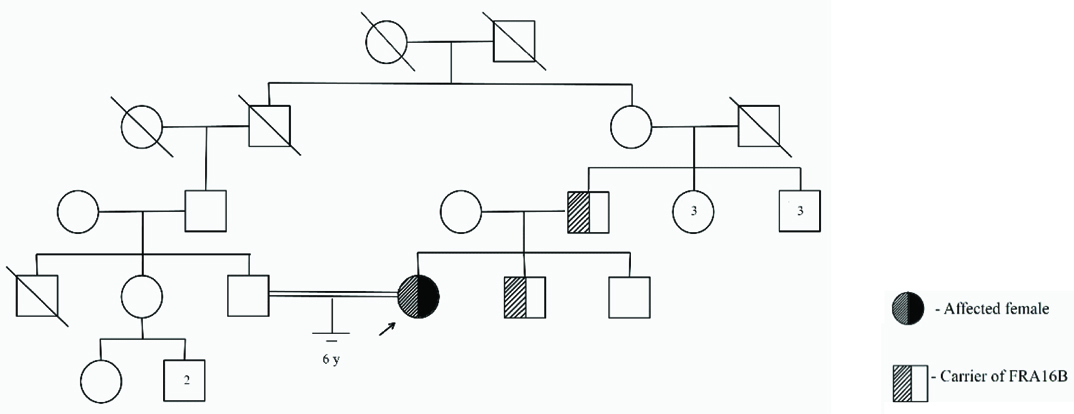
The 25-year-old proband who has married her second cousin [Table/Fig-1] presented with Secondary Amenorrhoea (SA). She gave a history of menarche at the age of 15 years, irregular cycles for four years and withdrawal bleeding with oral contraceptive pills for seven years. She exhibited moderately developed secondary sexual characters with retracted nipples. Abdominal Ultrasonography (USG) and pelvic Magnetic Resonance Imaging (MRI) scan showed a small and hypoplastic uterus which measured 1.4 cm in anteroposterior dimension and was anteverted [Table/Fig-2]. However, her ovaries could not be visualized. Pedigree analysis revealed no family history of infertility. The written informed consent was obtained from the patient.
Phenotypic characteristics of the proband.
| Features | Findings |
|---|
| Physical features | Height | 156 cm |
| Weight | 43 kg |
| Endocrine system | Thyroid | Normal |
| Secondary sexual characters | Breast development | Tanner IV |
| Axillary hair | Tanner IV |
| Pubic hair | Tanner IV |
| External genitalia | Normal |
| USG findings | Uterus | 37 mm x 11 mm x 27 mm |
| Right ovary | N.V† |
| Left ovary | N.V† |
| EM thickness | 1.8 mm |
| MRI pelvis | Uterus | Hypoplastic, anteverted |
| Right ovary | N.V† |
| Left ovary | N.V† |
| EM thickness | 1.5 mm |
| Hormonal profile | FSH (mIU/ml) | 79.54↑ | 1.12 |
| LH (mIU/ml) | 25.87↑ | 0.43↓ |
| Prolactin (ng/ml) | 16.77 | 46.93↑ |
| Total T3 (pg/ml) | 3.40 |
| Free T4 (ng/dl) | 1.21 |
| TSH (mIU/ml) | 2.18 |
;N.V- Not visualized;
↑ increased; ↓ decreased
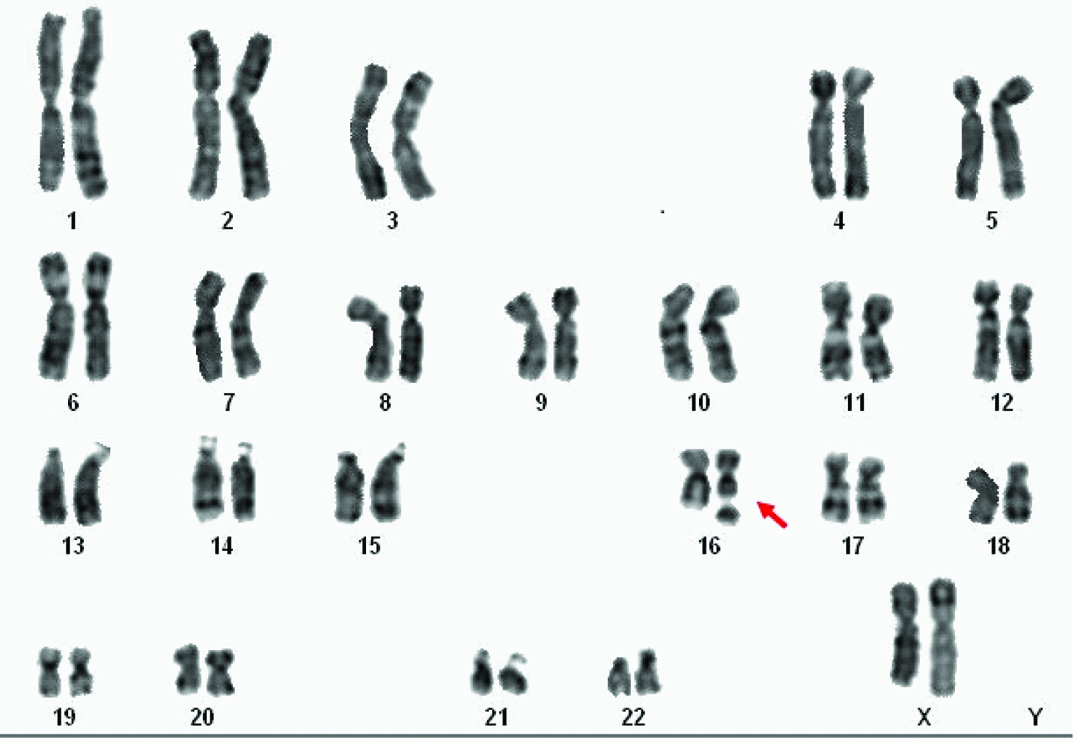
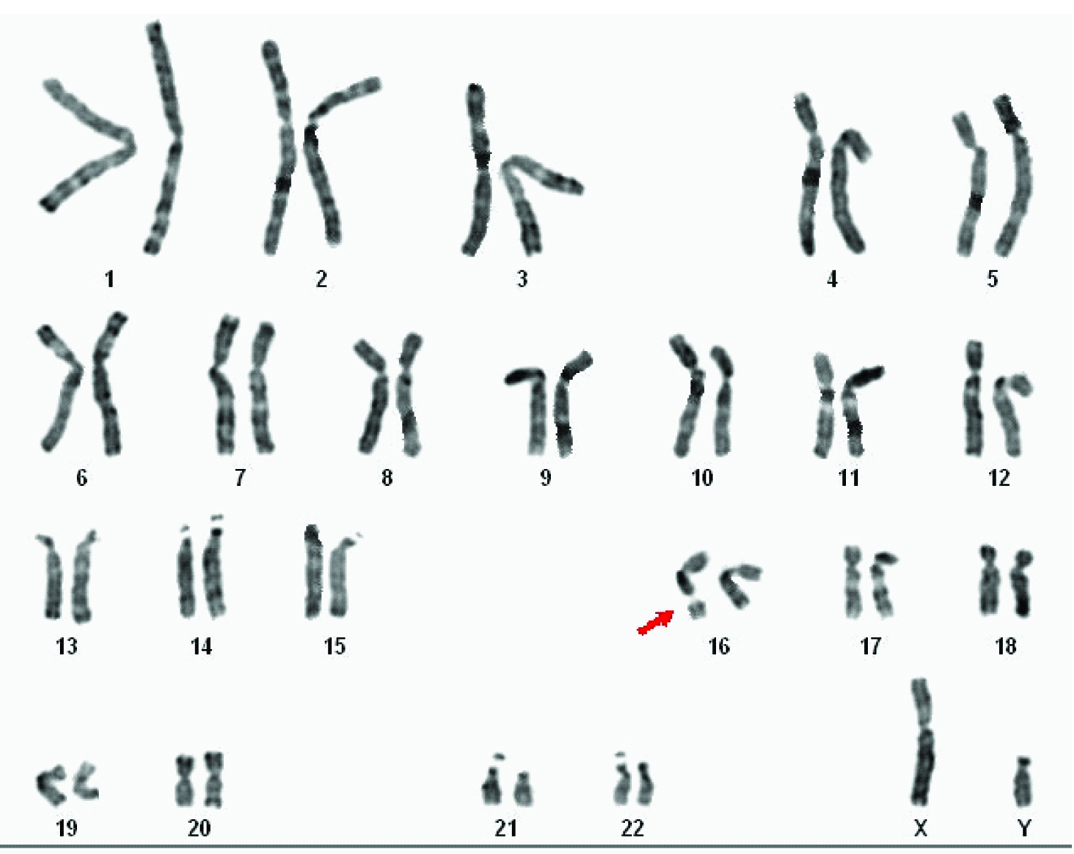
Routine chromosomal analysis revealed spontaneous expression of the Fragile Site (FS) FRA16B in the proband [Table/Fig-3a]. Subsequently parallel cultures were set up with addition of Berenil (30, 100 and 150 µg/ml), Hoechst 33258 (5 and 25 µg/ml) (Sigma-Aldrich) and 5-bromodeoxyuridine (BrdU) (30 and 50 µg/ml) (SRL) at different times prior to harvest, for her and her family members. Both GTG- and RHG-banded metaphases were analysed and the karyograms were prepared using ASI systems karyotyping software (BandView version 6.0). Chromosomal anomalies were designated following standard guidelines. FRA16B was also observed in her father and younger brother [Table/Fig-3b]. Her mother and elder brother exhibited normal karyotype in all cultures.
GTG-banded karyogram of proband.
(arrow indicates chromosome # 16 carrying the fragile site)
GTG-banded karyogram of the proband’s younger brother.
(arrow indicates FRA16B).
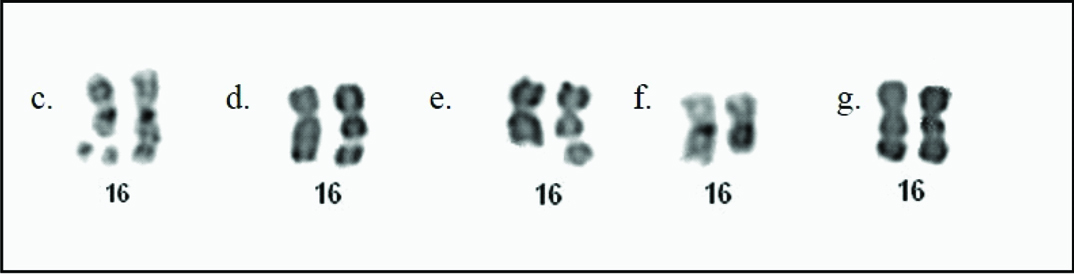
Fifty metaphases were scored for each treatment and the frequency of FRA16B was found to vary in all the three carriers. FRA16B was expressed as mono- or di-chromatid gaps/ breaks and more rarely as deletion or tri-radial configuration [Table/Fig-3c-f]. Homozygosity for FRA16B was observed in a single cell of her father [Table/Fig-3g]. While spontaneous expression of FRA16B was observed in few cells in the proband (7%), her father (3%) and younger brother (4%), addition of Berenil at 150 µg/ml, 24 hour prior to harvest revealed the maximum expression of FRA16B (78%, 86% and 56%) followed by 100 µg/ml (40%, 47%, 27%) and 30 µg/ml (15%, 7%, 7%) in the proband, her father and brother respectively. BrdU added at 30 µg/ml (after 67h of incubation) (14%, 40%, 50%) and at 50 µg/ml (64h) (22%, 21%, 20%) also increased FRA16B expression in the three individuals respectively. Likewise, Hoechst 33258 at 5 µg/ml aided in FRA16B induction (10%, 13%, 7%) but led to cell death at the higher dose. Thus, an optimal concentration of the additive and duration of exposure is crucial for enhanced expression of FS. The results were confirmed through replicate cultures.
Expression of FRA16B in cultured lymphocytes: c) Triradial configuration; d) Chromosome gap; e) Chromosome break; f) Deletion; g) Homozygosity for FRA16B.
Discussion
FS are unusual genomic breaks visible in metaphase chromosome preparations caused due to partial inhibition of DNA synthesis. Expansion of micro- or mini-satellite sequences has been attributed to underlie FS and in some cases to be responsible for inherited disorders. FS are often mapped to cancer-specific break points and to genes involved in tumorigenesis [1]. Based on their prevalence, they are classified as: i) Rare FS (RFS); and ii) Common FS (CFS). The RFS are further classified on the basis of the mode of induction as: i) folate-sensitive; ii) distamycin A-inducible; and iii) BrdU-requiring. The CFS may be induced by aphidicolin, 5-azacytidine or BrdU [1]. Chromosome 16 is the most unstable chromosome having ten FS, FRA16A to FRA16J and a FS occurs every 9.89Mb. The earliest report of the distamycin A-inducible FS on 16q22.1 (FRA16B) was in a large kindred with no apparent phenotypic effect. This region contains 33bp AT-rich repetitive sequences and around 7–12 copies are found in the normal allele, while in carriers the number increases up to 2000 [2]. Its expression is induced by AT-specific binding chemicals viz., distamycin-A, Hoechst 33258, Berenil, netropsin, 4’,6-diamidino-2-phenylindole (DAPI), interferon and methyl-green which bind externally and hinder DNA replication [1].
In our study, FRA16B expression was examined using additives viz., Berenil, Hoechst 33258 and BrdU. Of these chemicals Berenil is found to induce FRA16B expression in nearly 100% of the cells [3]. Distamycin A and Berenil share similar mechanism in inducing breaks in the carriers. Studies on the crystal structure of Berenil showed that it has preference for AT-rich sequences [4], and comparison studies of AT-specific binding chemicals showed that the affinity of Berenil is relatively higher, thereby causing an enhanced expression of FRA16B in the cultures [5]. The higher the dose of Berenil used the higher was the FS expression. This makes Berenil a potential chemical for routine diagnosis.
FRA16B is predominantly linked to disorders of reproduction, and the occurrence of dynamic mutation has been indicated as a possible mechanism. The plausible association of amenorrhoea and FRA16B appears to be enigmatic. A case of familial SA seemingly associated with paternally inherited fra(16)(q22) in three siblings was reported earlier [6]. The proband with clinical features of Turner syndrome exhibited a mosaic 45,X,fra(16)(q22)/46,XX,fra(16)(q22) karyotype while, two of her sisters with SA, short stature and hirsutism showed 46,XX,fra(16)(q22). The probable aetiological association of this FS with SA remains to be established in her sisters who showed a normal karyotype. The proband’s younger brother, a carrier of FRA16B was found to be normal in our study.
Reports linking FS on 16q with various reproductive disorders also do exist [Table/Fig-4]. Giraud F et al., Shabtai F et al., and Ghazaey S et al., described the likely association of FRA16B with spontaneous abortions, bilateral cryptorchidism, infertility, Klinefelter syndrome and severe oligozoospermia [7-11]. Aswini S et al., reported a non-consanguineous couple with recurrent abortions wherein both partners had expressed the fra(16)(q22) which is rather rare [2]. Martorell MR et al., described spontaneous expression of fra (16)(q22.1) in the male partner of an infertile couple in 50% of his cells [12]. Further investigation detected unbalanced anomalies of chromosome 16 in his sperm and in the embryos produced by In Vitro Fertilization-Preimplantation Genetic Diagnosis (IVF-PGD). The presence of FRA16B in either parent might increase the risk of de novo structural chromosome abnormalities in the progeny.
Reports of fra(16)(q22) in reproductive disorders in the literature.
| Proband’s features and karyotype | Associated disorder | Frequency of FS | Inheritance | Reference |
|---|
| Woman with 2 spontaneous abortions | BOH | 50% | - | Giraud F et al., [7] |
| Father of Turner syndrome child with 46,X,i(Xq) | | 11% | Paternal |
| Boy with obesity and bilateral cryptorchidism 45,X,FRA16/46,X,r(Y),FRA16 | DSD | 20% | - |
| Couple with spontaneous abortions | BOH | 12% in wife; 20% in husband | - | Shabtai F et al., [8] |
| Male with Klinefelter syndrome 47,XXY | DSD | 8% | Maternal |
| Severe oligozoospermic male 46,XY | Infertility | 10% | - |
| Severe oligozoospermic male with spastic bronchitis and asthma 46,XY | Infertility | 5% | Maternal | Shabtai F et al., [9] |
| Couple with spontaneous abortions and infertility | BOH | 5% (female) 5-20% in 4 males | - | Shabtai F et al., [10] |
| Proband – Short stature with SA 45,X,fra(16)(q22)/46,XX,fra(16)(q22)pat | Amenor- rhoea | 31.5% | Paternal | Krishna- murthy DS et al., [6] |
| Proband’s sib I - Short stature with SA 46,XX,fra(16)(q22)pat | 33.3% |
| Proband’s sib II- Short stature with SA 46,XX,ra(16)(q22)pat | 34.4% |
| Proband’s father | 35.3% | | - |
| Couple with spontaneous abortions | BOH | 35% in wife 45% in husband | Paternal (Wife) | Aswini S et al., [2] |
| Couple with infertility – Male carrier | Infertility | 50% | - | Martorell MR et al., [12] |
| Couple with spontaneous abortions | BOH | 3 cases | - | Ghazaey S et al., [11] |
*BOH- Bad obstetric history; DSD- Disorder of sex development
Association of FRA16B with miRNAs viz., hsa-mir-328, hsa-mir-6773, hsa-mir-1538, hsa-mir-140 and hsa-mir-1972-2 has been reported recently [13]. Analysis of miRò database [14] revealed the involvement of miR-140 in reproductive disorders such as recurrent pregnancy loss, pre-eclampsia, spontaneous preterm delivery and in cases of ovarian, breast and uterine cancer. Array-Comparative Genomic Hybridization (Array-CGH) analysis detected down-regulation of miR-328 in ovarian cancers. Menarche and menopause, ovulatory dysfunction, endometriosis, adenomyosis and leiomyomata, pregnancy loss, idiopathic azoospermia and male infertility are associated with hsa-miR-1972-2 indicating its causative role in reproductive disorders [14].
Appreciable data back-up on association of FRA16B with other disorders are also available. Co-segregation of FRA16B with aberrant orofacial development having features of microstomia, partial anodontia, dominant form of cleft palate and micrognathia has been reported [15]. FRA16B’s linkage with hypertrophic cardiomyopathy has also been found in the literature [16]. Carriers often exhibiting chromosome rearrangements, mostly pericentric inversions, involving band 16q22 were found to carry an increased risk of predisposition to acute myelomonocytic leukaemia. The increased fragility observed at FS is in a way linked to the genetic instability inherent in transformed cancer cells [17].
Dynamicity of the repetitive sequences from normal to premutation to full mutation through successive generations accounts for FS expression. These full mutations can inactivate genes and implicate regions of genomic instability. Glasser L et al., speculated FRA16B to be a factor for development of myelodysplastic syndrome and acute myeloid leukaemia in a case of familial benign neutropenia [18]. Both the mother and her daughter exhibited fra(16)(q22) along with del(16)(q22). Genetic anticipation was suggested as the frequency of the FS in the daughter was higher. FRA16B segregates as a dominant, completely penetrant phenotype and its inheritance is mostly maternal but paternal inheritance was observed in our case.
Conclusion
In conclusion, reports of additional families with similar phenotypes co-segregating with the FS would help in understanding the role of FRA16B in amenorrhoea. Further screening of this FS under induced conditions in other family members might help to identify yet undiagnosed conditions in the carriers.
†;N.V- Not visualized;
↑ increased; ↓ decreased
*BOH- Bad obstetric history; DSD- Disorder of sex development
[1]. Durkin SG, Glover TW, Chromosome fragile sitesAnnu Rev Genet 2007 41:169-92. [Google Scholar]
[2]. Aswini S, Jegatheesan TN, Chandra N, Spontaneous expression of FRA16B in a non-consanguineous couple experiencing multiple fetal lossesJ Obstet Gynaecol Res 2012 38:1223-27. [Google Scholar]
[3]. Sutherland GR, Rare fragile sitesCytogenet Genome Res 2003 100:77-84. [Google Scholar]
[4]. Martins-Jose A, Sliding box docking: a new stand-alone tool for managing docking-based virtual screening along the DNA helix axisBioinformation 2013 9(14):750-51. [Google Scholar]
[5]. Schmid M, Feichtinger W, Jebberger A, Kohler J, Lange R, The fragile site (16) (q22) I. Induction by AT-specific DNA ligands and population frequencyHum Genet 1986 74:67-73. [Google Scholar]
[6]. Krishnamurthy DS, Naguib KK, Al-Awadi SA, Sundareshan TS, Al-Othman SA, Hayat AA, Clinical and cytogenetic studies in familial polycystic ovarian diseaseJ Obstet Gynaecol 1989 10:133-39. [Google Scholar]
[7]. Giraud F, Ayme S, Mattei JF, Mattei MG, Constitutional chromosomal breakageHum Genet 1976 34:125-36. [Google Scholar]
[8]. Shabtai F, Bichacho S, Halbrecht I, The fragile site on chromosome 16 (q21q22) Data on four new familiesHum Genet 1980 55:19-22. [Google Scholar]
[9]. Shabtai F, Klar D, Bichacho S, Hart J, Halbrecht I, Familial fragility on chromosome 16 (Fra 16q22). Enhanced by both interferon and distamycinA. Hum Genet 1983 63:341-44. [Google Scholar]
[10]. Shabtai F, Orlyn J, Hart J, Bichacho S, Halbrecht I, Alpha-interferon and fragility at 16q22- A study on 15 selected controls and 146 selected patientsHum Genet 1987 75:48-52. [Google Scholar]
[11]. Ghazaey S, Keify F, Mirzaei F, Maleki M, Tootian S, Ahadian M, Chromosomal analysis of couples with repeated spontaneous abortions in northeastern IranInt J Fertil Steril 2015 9(1):47-54. [Google Scholar]
[12]. Martorell MR, Martinez-Pasarell O, Lopez O, Polo A, Sandalinas M, Garcia-Guixe E, Chromosome 16 abnormalities in embryos and in sperm from a male with a fragile site at 16q22.1Cytogenet Genome Res 2014 142:134-39. [Google Scholar]
[13]. Georgakilas GA, Tsantoulis P, Kotsinas A, Michalopoulos I, Townsend P, Gorgoulis VG, Are Common fragile sites merely structural domains or highly organized ‘functional’ units susceptible to oncogenic stressCell Mol Life Sci 2014 71:4519-44. [Google Scholar]
[14]. Laganà A, Forte S, Giudice A, Arena MR, Puglisi PL, Giugno R, miRò: a miRNA knowledge baseDatabase (Oxford) 2009 2009:bap008 [Google Scholar]
[15]. McKenziea F, Turner A, Withers S, Dalzell P, McGlynne M, Kirkb EPE, Dominant inheritance of cleft palate, microstomia and micrognathia-possible linkage to the fragile site at16q22 (FRA16B)Clin Dysmorphol 2002 11:237-41. [Google Scholar]
[16]. Marian AJ, Roberts R, Recent advances in the molecular genetics of hypertrophic cardiomyopathyCirculation 1995 92:1336-47. [Google Scholar]
[17]. Weckerle AB, The molecular basis of fragile site FRA16B and its role in Inv(16) (p13q22) in acute myeloid leukaemia [Ph.D.] 2011 North CarolinaWake Forest University Graduate School of Arts and ScienceAvailable from: http://hdl.handle.net/10339/33465 [Google Scholar]
[18]. Glasser L, Meloni-Ehrig A, Joseph P, Mendiola J, Benign chronic neutropenia with abnormalities involving 16q22, affecting mother and daughterAm J Hematol 2006 81:262-70. [Google Scholar]