Spotted fever, scrub typhus and epidemic typhus jointly known as rickettsioses, a group of vector borne infectious diseases caused by different species of the small Gram-negative obligate intracellular bacteria Rickettsia and Orientia [1]. Spotted fever rickettsiosis consists of Indian tick typhus, Rocky Mountain spotted fever and Rickettsial pox caused by Rickettsia conorii, R. rickettsii and mite transmitted R. akari. In India, commonly reported rickettsial diseases are Indian tick typhus, murine flea-borne typhus, scrub typhus and Q fever [2]. Fever, headache, myalgia and malaise are the initial clinical symptoms of rickettsioses so it is hard to distinguish it from other infectious and non infectious diseases. So, the big challenge to clinician is the tough diagnostic predicament posed by these rickettsial infections initially in their clinical procedure when antibiotic therapy is most effective [3,4]. Extreme appearances incorporate protracted fever, renal disappointment, myocarditis, meningoencephalitis, hypotension, intense respiratory pain disorder and various organ disappointments [3]. Disappointment of opportune conclusion prompts to noteworthy grimness and mortality. With convenient finding, treatment is simple, moderate and regularly fruitful with emotional reaction to antimicrobials. The illness happens overwhelmingly in Mediterranean territories, for example, India and Africa. In India, it has been clearly reported from several states including Himachal Pradesh, Tamil Nadu, Kerala, Maharashtra, Bihar, Karnataka, Jammu and Kashmir, Uttaranchal, Rajasthan, West Bengal and Meghalaya [5-7] and recently we have evidently reported four cases of SFR from Uttar Pradesh [1] which showed a need for detailed studies to determine the burden and epidemiology of SFR in these areas so that effective control measures could be implemented. To be clinically helpful as an indicative tool, affectability should be adequately high in the early phase of malady to bolster particular analysis driven treatment. The Weil-Felix agglutination test, popularized by the Indian army in the 1930s, continues to be the only test available in most medical institutions in India [4]. Poor affectability and specificity of the Weil-Felix test is currently all around showed and thus is utilized just as a first line of testing in simple healing facility research centers. In the present study, samples from patients were tested by Weil felix test for the presence of antirickettsial antibodies followed by ELISA and IFA test for specific IgM antibody detection against Rickettsia conorii infection to enhance the diagnosis and epidemiology in the state of Uttar Pradesh.
Materials and Methods
This was a prospective study (hospital based surveillance) and the study population included individuals of all age groups, who attended the Outpatient and Inpatient Departments of King George’s Medical University, Lucknow, Uttar Pradesh, India and its associated hospitals with a diagnosis of Pyrexia of Unknown Origin (PUO) or experiencing a febrile illness clinically consistent with rickettsiosis infection during May 2013 to February 2015. A questionnaire was designed to note down the demographic details (so that follow up of patient is easy for collection of paired sera) and clinical findings of the suspected patient. The protocol and study with patients was approved by the Ethics committee of the King George’s Medical University, Lucknow, Uttar Pradesh, India (approval no. 7215/ Ethics/R. Cell-15) and written informed consent was obtained from patients before enrolment to this study. All the subjects underwent clinical examination by the clinician for rickettsial and other possible infections. Three ml each of whole blood samples collected in plane vial and serum was separated and aliquoted. All samples were stored in duplicate at -80°C for future analysis. When available, convalescent paired sera were also tested to enhance the diagnosis sensitivity. Each sample was tested for Rickettsia specific tests viz. ELISA and IFA for Rickettsia conorii IgM antibodies and Weil felix test for all the three antigens OXK, OX2 and OX19 using commercial kits (Vircell Microbiologists, Spain for ELISA, Fuller Laboratories, Fullerton, California for IFA and Tulip Diagnostics, Goa for Weil Felix test) following manufacturer’s protocol.
Antigens Proteus vulgaris 0X2, P. vulgaris 0X19 and P. mirabilis OXK were obtained commercially from Tulip Diagnostics, Goa, India. Weil Felix test was done using manufacturer’s protocol. In brief, the smooth, executed recolored antigen suspensions were blended with the patient’s serum. Antibodies created because of rickettsial contamination if display in the patient serum was respond with the recolored antigen suspension to deliver an agglutination response. No agglutination shows the nonattendance of rickettsial antibodies. Samples were tested using Rickettsia conorii ELISA IgM kit (Vircell Microbiologists, Spain) as per manufacturer’s protocol. In brief, 25 µl of sorbent was added to each of the required wells prior to the addition of serum diluents, then patient sera and control sera (positive, negative and cut-off) were used at a dilution of 1:100 using serum diluent and kept for 45 minutes at 37°C. An anti human IgG peroxidise conjugate dilution in an orange coloured Proclin-containing buffer was added for 30 minutes at 37°C. Finally the substrate solution containing tetramethylbenzidine was added for 20 minutes at room temperature and the plate was read at 450/620 nm within one hour of stopping.
Weil Felix and Rickettsia conorii IgM ELISA tests were translated by the producer’s rules. A titre of over 1:80 was considered as positive for OXK, OX2 and OX19 antigens for Weil Felix test and antibody index greater than 11 considered positive for IgM antibody. The antibody index was calculated in comparison to the cut off serum control by following formula -
Antibody index = (Sample O.D. /cut off serum mean O.D.) × 10
Samples were tested using Rickettsia conorii/Rickettsia typhi IFA IgM Antibody kit (Fuller Laboratories, California, USA) as per manufacturer’s protocol. In brief, serially 2-fold dilutions of the positive control were prepared in wash buffer to incorporate one dilution above and one dilution beneath the expressed endpoint (1:512). Then, 10 µl dilutions of test serum were added to each slide wells and for each set of slides, the negative control and dilutions of the positive controls were included. Slides were then placed into a humidity chamber for incubation at 37°±0.5°C. After incubation, slide wells were rinsed with wash buffer three times. Then goat anti-mouse IgM (heavy chain) and goat anti-mouse IgG conjugate were added to each slide well for 30 minutes at 37°±0.5°C. Finally, 2-3 drops mounting medium were added to each slide and the slide was read using fluorescence microscope with 400 X magnification and channel framework for FITC (most extreme excitation wavelength 490 nm, mean outflow wavelength 530 nm).
A positive response shows up as bright expression (no less than 1+) when compared with the positive and negative control reactions. Patterns of reactivity different from the positive controls was considered non specific. Differential diagnosis of malaria (antigen detection, Optimal, Bio-Rad Laboratories India Pvt., Ltd., Gurgaon, Haryana, India), dengue (NS1 antigen, Microlisa, J. Mitra and Co. Pvt., Ltd., New Delhi, India), typhoid (Typhidot, AB Diagnopath Manufacturing Pvt., Ltd, New Delhi, India; Widal; in-house) and hepatitis (Merilisa HBsAg, Meril Diagnostics Pvt., Ltd, Vapi, Gujarat, India) etc., was performed using commercial kits following manufacturer’s protocol to see the associated coinfections, if any.
The patients were subjected to battery of investigations such as haemogram, Liver Function Tests (LFT), Kidney Function Tests (KFT), bleeding and clotting time, prothrombin time/ International Normalized Ratio (INR), activated partial thromboplastin time, chest X-Ray, fibrin split products, stomach ultrasonography and so on wherever important to prove the diagnosis.
Statistical Analysis
The data was statistically analysed on Graph Pad Prism (5.0) software by using Chi-square test. In statistical analysis by Chi-square test, the probability value (p-value) of less than 0.05 was considered significant.
Results
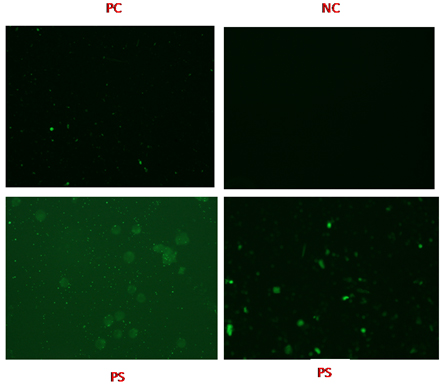
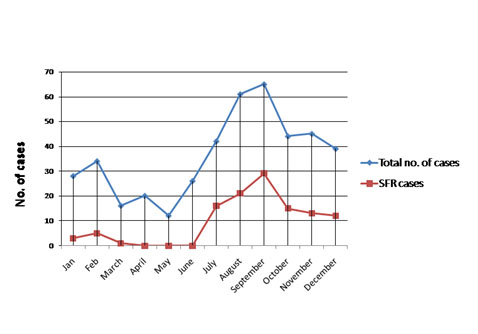
There were 432 patients, whose serum samples were collected during the study period. Among those, 194 samples were from outpatients and 238 samples were from patients admitted in various wards of the hospital on the basis of PUO where routine examinations were unremarkable and rickettsial illness was kept as one of the differential investigation. Clinical data were noted on pre designed questionnaire. Blood samples were collected as mentioned above and sera were stored at -20°C till tested. Of the 432 blood samples tested by Weil felix test, 200 (46.29%) showed titre 1:80 or more and were considered as positive. However, when these samples were also tested by ELISA and IFA against Rickettsia conorii IgM antibody to confirm the diagnosis, as these are more specific diagnostic tests, only 115 (26.62%) samples were found to be positive and these samples were also positive by Weil felix. Immunofluorescence images of the Positive Control (PC), Negative Control (NC) and positive Patient Samples (PS) are depicted in [Table/Fig-1]. Age and sex wise distribution of PUO cases are depicted in [Table/Fig-2]. Out of the 432 samples, 234 (54.2%) were males and 198 (45.8%) were females. All positivity for SFR was significantly (p<0.05) slightly higher (26.8%) among females who were suffering from PUO in comparison to males (26.5%). Among females, highest positivity was seen in 0-15 (40.8%) years of age group and it was same in both 31-45 (20%) and 46-60 (20%) years of age group followed by 16-30 (19.2%) and >60 (10%) years of age group. In males, highest positivity was also seen in 0-15 years (43.5%) followed by 46-60 (17.2%), 31-45 (15.4%), 16-30 (14.9%) and >60 (14.3%) years of age groups respectively. On statistical analysis, the difference in positivity for SFR in different age group of both male and female was significant (p<0.05). Most of the patients were from rural areas belonging to different districts of Uttar Pradesh [Table/Fig-3]. Regarding occupation, most of our patients were farmers and self- employed. Maximum numbers of cases were seen mainly in the months between July to December [Table/Fig-4]. Fever was the most common manifestation (~75%) among all the age groups. The other symptoms noted were rashes (53.7%), lymphadenopathy (46.9%), hepatomegaly (45.5%), nausea (45.8%), cyanosis (39.5%), thrombocytopenia (36.8%), pallor (32.8%), myalgia (32.5%), CNS symptom (30.4%) followed by headache (28.0%), bleeding (27.7%), oedema (25.5%), abdominal pain (25.1%) splenomegaly (25.1%), icterus (22.6%) and anasarca (22.5%). The pathognomonic features such as eschar were rare in the present study; only four patients (3.4%) had the presence of eschar [Table/Fig-5]. Some of the pictures of the SFR patients in our study are depicted in [Table/Fig-6].
Immunofluorescence images of the positive control (PC), negative control (NC) and positive patient samples (PS).
Age and sex distribution of pyrexia of unknown origin (PUO) cases. The data was statistically analysed using Chi-square test and the p-value of less than 0.05 was considered significant
| Age group (years) | Male | Female |
|---|
| Total | Positive (%) | Negative (%) | Total | Positive (%) | negative (%) |
|---|
| 0-15 | 92 | 40 (43.5) | 52 (56.5) | 71 | 29 (40.8) | 42 (75) |
| 16-30 | 47 | 7 (14.9) | 40 (85.1) | 52 | 10 (19.2) | 42 (82.9) |
| 31-45 | 52 | 8 (15.4) | 44 (84.6) | 40 | 8 (20) | 32 (86.9) |
| 46-60 | 29 | 5 (17.2) | 24 (82.8) | 25 | 5 (20) | 20 (90) |
| >60 | 14 | 2 (14.3) | 12 (85.7) | 10 | 1 (10) | 9 (100) |
| Total | 234 | 62 (26.5) | 172 (73.5) | 198 | 53 (26.8) | 145 (73.2) |
| | Chi-square (X2) = 22.52 Degree of Freedom (DOF) = 4 p value < 0.05 | | | Chi-square (X2) = 11.64 Degree of Freedom (DOF) = 4 p value < 0.05 | |
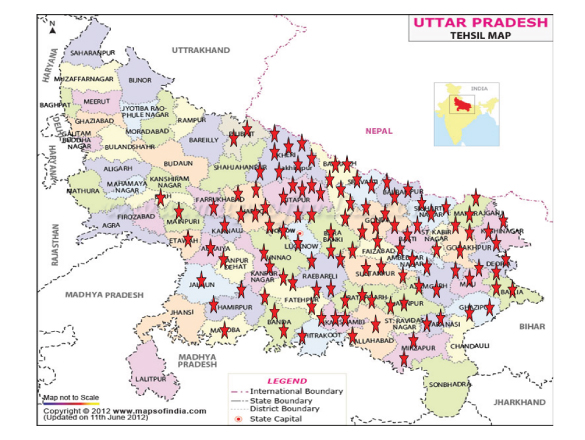
Distribution of SFR cases in U.P (Asterisks in red represents the number of patients in that area).
Distribution of total and SFR cases around the year.
Clinical features and signs of the patients. The data was statistically analysed using Chi-square test and the p-value of less than 0.05 was considered significant.
| CLINICAL FEATURES and SIGNS | WEIL FELIX POSITIVE | wEIL FELIX NEGATIvE | p-value | IgM ab + (both from Elisa and ifa) | IgM Ab - (both from ELISA and I FA) | p-value |
|---|
| FEVER GRADE | HIGH | 129 (61.1%) | 82 (38.9%) | 0.0916 | 68 (31.5%) | 148(68.5%) | 0.0724 |
| MODERATE | 47 (52.2%) | 43 (47.8%) | | 29 (22.1%) | 102(77.9%) | |
| MILD | 24 (46.2%) | 28 (53.8%) | | 18 (21.2%) | 67 (78.8%) | |
| FEVER DURATION | Fever <7days | 33 (31.4%) | 72 (68.6%) | 0.0011 | 26 (26.5%) | 72 (73.5%) | 0.8756 |
| Fever 7-13 days | 76 (57.6%) | 56 (42.4%) | | 44 (30.8%) | 99 (69.2%) | |
| Fever 14-29 days | 40 (46.5%) | 46 (53.5%) | | 24 (27.9%) | 62 (72.1%) | |
| Fever > 30 days | 51 (46.8%) | 58 (53.2%) | | 21 (20.0%) | 84 (80.0%) | |
| RASH | PRESENT | 102 (61.4%) | 64 (38.6%) | <0.0001 | 86 (53.7%) | 74 (46.2%) | <0.0001 |
| ABSENT | 98 (36.8%) | 168 (63.2%) | | 29 (10.6%) | 243(89.3%) | |
| HEADACHE | PRESENT | 109 (46.8%) | 124 (53.2%) | 0.8269 | 63 (28.0%) | 162(72.0%) | 0.4988 |
| ABSENT | 91 (45.7%) | 108 (54.3%) | | 52 (25.1%) | 155(74.9%) | |
| NAUSEA/VOMITING | PRESENT | 128 (51.8%) | 119 (48.2%) | 0.0078 | 74 (45.8%) | 98 (54.2%) | <0.0001 |
| ABSENT | 72 (38.9%) | 113 (61.1%) | | 41 (19.9%) | 219(80.1%) | |
| MYALGIAS | PRESENT | 108 (50.7%) | 105 (49.3%) | 0.0700 | 78 (32.5%) | 162(67.5%) | 0.0020 |
| ABSENT | 92 (42.0%) | 127 (58.0%) | | 37 (19.3%) | 155(80.7%) | |
| ABDOMINAL PAIN | PRESENT | 97 (44.9%) | 119 (55.1%) | 0.5626 | 59 (25.1%) | 176(74.9%) | 0.4368 |
| ABSENT | 103 (47.7%) | 113 (52.3%) | | 56 (28.4%) | 141(71.6%) | |
| BLEEDING | PRESENT | 64 (47.4%) | 71 (52.6%) | 0.7548 | 18 (27.7%) | 47 (72.3%) | 0.8320 |
| ABSENT | 136 (45.8%) | 161 (54.2%) | | 97 (26.4%) | 270(73.6%) | |
| ANASARCA | PRESENT | 86 (44.1%) | 109 (55.9%) | 0.4068 | 54 (22.5%) | 186(77.5%) | 0.0303 |
| ABSENT | 114 (48.1%) | 123 (51.9%) | | 61(31.8%) | 131(68.2%) | |
| CNS SYMPTOM | PRESENT | 79 (48.5%) | 84 (51.5%) | 0.4814 | 42 (30.4%) | 96 (69.6%) | 0.2191 |
| ABSENT | 121 (45.0%) | 148 (55.0%) | | 73 (24.8%) | 221(75.2%) | |
| PALLOR | PRESENT | 91 (46.4%) | 105 (53.6%) | 0.9599 | 45 (32.8%) | 92 (67.2%) | 0.0460 |
| ABSENT | 109 (46.2%) | 127 (53.8%) | | 70 (23.7%) | 225(76.3%) | |
| CYANOSIS | PRESENT | 98 (47.3%) | 109 (52.7%) | 0.6756 | 64 (39.5%) | 98 (60.5%) | <0.0001 |
| ABSENT | 102 (45.3%) | 123 (54.7%) | | 51 (18.9%) | 219 (81.1%) | |
| OEDEMA | PRESENT | 115 (50.7%) | 112 (49.3%) | 0.0556 | 61 (25.5%) | 178(74.5%) | 0.5658 |
| ABSENT | 85 (41.5) | 120 (58.5%) | | 54 (28.0%) | 139(72.0%) | |
| ICTERUS | PRESENT | 89 (46.4%) | 103 (53.6%) | 0.9828 | 65 (22.6%) | 223(74.4%) | 0.0071 |
| ABSENT | 111 (46.3%) | 129 (53.8%) | | 50 (34.7%) | 94 (65.3%) | |
| ESCHAR | PRESENT | 12 (26.1%) | 34 (73.9%) | 0.0036 | 4 (21.1%) | 15 (78.9%) | 0.0002 |
| ABSENT | 188 (48.7%) | 198 (51.3%) | | 111 (26.9%) | 302 (73.1%) | |
| THROMBOCYT OPENIA | PRESENT | 92 (60.9%) | 59 (39.1%) | <0.0001 | 88 (36.8%) | 151(63.2%) | <0.0001 |
| ABSENT | 108 (38.4%) | 173 (61.6%) | | 27 (14.0%) | 166 (86.0%) | |
| LYMPHADEN OPATHY | PRESENT | 118 (53.6%) | 102 (46.4%) | 0.0018 | 91 (46.9%) | 103 (53.1%) | <0.0001 |
| ABSENT | 82 (38.7%) | 130 (61.3%) | | 24 (10.1%) | 214 (89.9%) | |
| HEPATOMEGALY | PRESENT | 128 (56.6%) | 98 (43.4%) | <0.0001 | 96 (45.5%) | 157(54.5%) | <0.0001 |
| ABSENT | 72 (34.9%) | 134 (65.1%) | | 19 (11.4%) | 160(88.6%) | |
| SPLENOMEGALY | PRESENT | 81 (39.1%) | 126 (60.9%) | 0.0042 | 49 (25.1%) | 146 (74.9%) | 0.5244 |
| ABSENT | 119 (52.9%) | 106 (47.1%) | | 66 (27.8%) | 171(72.2%) | |
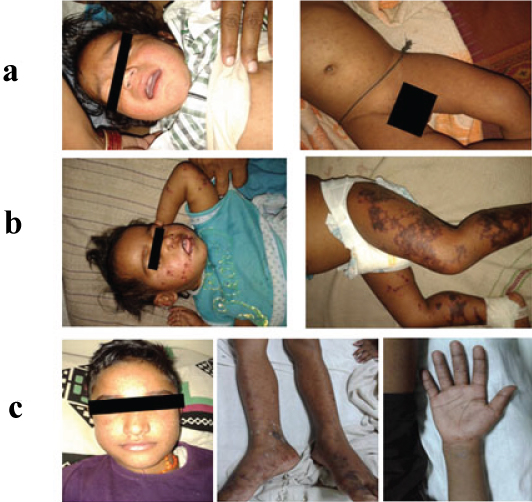
Some of the pictures of the SFR patients in our study a) Macular rashes on face and on whole body; b) Reticulated rashes on face and on leg; c) Maculopapular rash on face, reticulated rash on sole and on palm.
Among the laboratory investigations, raised transaminases (79.1%) [mean SGOT(aspartate transaminase)/SGPT (alanine transaminase)] were 254/135 IU/l), leukocytosis (73.9%), alkaline phosphatase (~68.6%) patients (mean 182.3 IU/l), low serum albumin (61.7%) and raised serum urea (60.0%) were main findings [Table/Fig-7]. We also found that 31 patients with rickettsial infection also had associated coinfections like typhoid, malaria, dengue and hepatitis etc. In the present study, all patients were empirically treated with injection ceftriaxone and amikacin. Once serology reports were available on day two, patients were treated with doxycycline empirically in the dosage of 100 mg twice a day for 10 days on the clinical suspicion of SFR. There was rapid improvement in patient’s condition and most of them got to be distinctly afebrile within 24-48 hours and were released within next 7-9 days; except for nine patients who presented late to hospital with multiple organs dysfunction and did not survive (mortality rate 7.80%).
Laboratory investigations of SFR cases (values in parentheses are percentages).
| Laboratory Investigations | No. of patients n= 115 (%) |
|---|
| Low haemoglobin(<9.0 gm%) | 46 (40.0) |
| Leucocytosis (>12, 000/μl) | 85 (73.9) |
| Raised bilirubin (>1.2 mg%) | 49 (42.6) |
| Raised SGPT/SGOT (>40 IU/l) | 91 (79.1) |
| Raised alkaline PO4 (>130 IU/l) | 79 (68.6) |
| Raised serum creatinine (>1.4 mg%) | 48 (41.7) |
| Raised serum urea (>45 mg/dl) | 69 (60.0) |
| Low serum albumin (<3.0 gm%) | 71 (61.7) |
| Low blood pressure (<100/60 mm Hg) | 46 (40.0) |
| High respiratory rate (>40 /minute) | 37 (32.1) |
| High pulse rate (>100 /minute) | 39 (33.9) |
Discussion
SFR has been reported from various parts of the country in the recent past with serological evidence of widespread occurence of SFR in the neighbouring states as shown in [Table/Fig-8] [1,8-10]. Thus, there was a strong possibility of presence of SFR in Uttar Pradesh also but no information was available yet. In the present study we surprisingly are detailing a pervasiveness of about 27% of SFR in clinically speculated patients revealing at a tertiary care centre in Uttar Pradesh. This is somewhat lower than what has been reported from other parts of the country [11-13]. The occurrence of SFR varied with age, gender and occupation. Our results showed that the incidence of infection was almost same in both male and female paediatric age group, possibly due to increased exposure as a result of a propensity for outdoor activities which is in contrast to what has been reported by other authors [14]. Most of the patients were from rural background as reported from other places of India [11,15]. Most of the cases were reported during the months of August to December. This coincides well with the active period of ticks and mites; during or at the end of rainy season. Similar period of disease occurrence is reported earlier also [16].
Comparison of the present study with different studies across India.
| Parameters | Present study | Other studies |
|---|
| Occurence of SFR | Uttar Pradesh | Uttar Pradesh; Singh M et al., 2015 [1] Jammu and Kashmir, Himachal Pradesh, Uttaranchal, Rajasthan, Assam, West Bengal, Maharashtra, Pondichery, Kerala and Tamilnadu; Mahajan SK et al., 2012 [8] Mumbai; Shah V et al., 2009 [9] Haryana; Chaudhry D et al., 2009 [10] |
| Sero prevalence of spotted fever rickettsiosis | 26.6% | 27.5%; Mittal V et al., 2012 [11] 12.1%; Ajantha GS et al., 2013 [12] 32.3%; Rathi NB et al., 2011 [13] |
| Age Group | 0-15 years | 11-60 years; Mittal V et al., 2012 [11] 30-45 years; Ajantha GS et al., 2013 [12] 2 months-20 years; Rathi NB et al., 2011 [13] 0-15 years; Bithu R et al., 2014 [14] |
| Patients background | Rural | Rural; Mittal V et al., 2012 [11] Rural; Wei Y et al., 2014 [15] |
| Season | During early cooler months | Early cooler months; Sharma et al., 2005 [16] |
| Common presenting symptom | Fever | Fever with chills; Mittal V et al., 2012 [11] Rash; Rathi NB et al., 2011 [13] Fever; Munilakshmi P et al., 2015 [19] Rash; Liyanapathirana et al., 2011 [20] |
| Eschar | Present | Present; Liyanapathirana VC et al., 2011 [20] Present; Paris DH et al., 2013 [21] |
| Lymphadenopathy | 50% | Common; Mahajan SK et al., 2008 [23] Common; Mahajan SK et al., 2006 [24] |
| Serum transaminases | Rise | Rise; Wei Y et al., 2014 [15] Rise; Kamarasu K et al., 2007 [22] Rise; Varghese GM et al., 2006 [25] |
| Tests performed | Weil Felix, ELISA, IFA | Weil Felix; Mittal et al., 2012 [11] Weil Felix; Ajantha et al., 2013 [12] Weil Felix, ELISA; Rathi et al., 2011 [13] |
| Co-infection | Typhoid, Malaria, Dengue and Hepatitis | Hepatitis, Typhoid, Dengue; Ajantha et al., 2013 [12] |
| Paired serology | Possible | Possible; Mittal V et al., 2012 [11] |
| Mortality rate | 7.80% | 9%; Rathi NB et al., 2011 [13] 14%; Varghese GM et al., 2006 [25] 6.1%; Lee CS et al., 2009 [27] 34%; Vivekanandan M et al., 2010 [28] |
Rickettsiosis shows as an intense febrile ailment with non-particular signs and symptoms [17]. In the present study, the most common clinical manifestation seen was that of fever (~75%) with rash (54%) as reported from other places of India as well [11,13,18-20]. Establishing the aetiological diagnosis is difficult during the acute stage of illness and the clinical features may be confused with atypical measles, typhoid, dengue, malaria and hepatitis. We also found that 31 patients with rickettsial infection also had associated co-infections like typhoid, malaria, dengue and hepatitis. It has been reported earlier also [12]. Hence even if a patient tests positive for the more common endemic infections prevalent in our setup, we must test for rickettsiosis so that appropriate treatment could be administered. An eschar at the site of inoculation, can be found in a highly variable (7-97%) percentage of people [21], however, it is rarely seen in south East Asia and Indian subcontinent [16,22]. In our study, eschar was seen in only four patients. Though lymphadenopathy is common in rickettsioses [23,24]; however, in the present study, nearly half of the patients showed this sign.
Similar to other studies [15,22,25]; most patients had rise of serum transaminases, even with no other proof of multiorgan brokenness. Other laboratory investigations recorded were leukocytosis, hepatomegaly, splenomegaly, thrombocytopenia, and low serum albumin. While low serum albumin and leucocytosis are thought to be related with serious rickettsiosis [16,26]. The mainstay of diagnosis in rickettsial infections is serology [27]. The cheapest test currently available and used extensively in our country [22,28,29] is Weil- Felix test which is highly specific, but lacks sensitivity. The test shows false negative results in the early stage of disease as the agglutinating antibodies can be detected only in the second week of illness. The IgM ELISA has good sensitivity and specificity [23,27,30], it is easy to perform, gives swift results and is suitable for testing large number of specimens; hence it may be considered as good replacement for Weil-Felix test for diagnosis of SFR. The gold standard serological tests are immunoflorescence IgG/IgM antibody test or indirect immunoperoxidase assay [26] but are out of reach in our country owing to high costs and lack of expertise. In the present study, we used initially Weil felix test for rickettsial antibodies followed by ELISA and IFA testing for specific IgM antibodies against R. conorii for diagnosis. In the present study, the most common organ dysfunction was hepatomegaly, splenomegaly followed by gastrointestinal disfunction and mutiorgan failure. Authoritative diagnosis usually requires uplifted clinical doubt and examination of convalescent serum samples for serological proof [31]. Paired serology was possible in most of the IPD positive cases and all of these exhibited greater than 2-3 fold ascend in titre in Weil Felix test 6-8 days separated. Here we have reported most of the confirmed cases of SFR where clinical doubt took after by examination of convalescent serum samples for serological proof, affirmed the diagnosis and incite treatment prompted to recuperation in most of the patients. In our study, nine patients succumbed to disease (mortality rate 7.80%). This is moderately lower when contrasted with other studies [25,27,28]. All patients who succumbed had presented with multiorgan brokenness; of these, six had not gotten antibiotic medications or macrolides. In other three patients, doxycycline was started late (second week of disease).
Limitation
The limitation of this study is that PCR (a more confirmatory marker) for diagnosis of SFR was not possible as PCR is a very costly test. This is a hospital based study which has its own impediments; hence future research can be done with community-based study to get more information on sero prevalance of SFR in Uttar Pradesh.
Conclusion
The results of the present study will have major contribution towards providing information on prevalence of rickettsial infections in the community so that effective control measures can be implemented. Delay in treatment may lead to complications and higher mortality. This will also give a unique opportunity to validate clinical signs and symptoms of rickettsial infections and agreement between clinically suspected and laboratory proven cases. Further this will evaluate utility of serodiagnosis in our set up and for early and reliable detection of ‘difficult-to-diagnose’ and ‘easy-to-treat’ rickettsioses pathogens.