Globally, there is a rapid increase in incidence and prevalence of diabetes mellitus making it as a global health emergency. Currently, 415 million adults are with diabetes and this number is expected to reach 642 million by 2040 [1]. This massive prevalence of diabetes has a major impact on the emergence of the associated complications and reduced quality of life, disability, and premature death [1].
Diabetes is the leading cause of Chronic Kidney Disease (CKD) and End Stage Renal Disease (ESRD). Published evidence highlights that almost 40% patients with diabetes develop kidney damage [2]. In US, the prevalence of CKD in patients with T2DM from 2007 to 2012 remained constantly high. The recently reported prevalence of CKD in T2DM was 58.7% and 25.7% in patients aged ≥65 years and <65 years respectively [3]. A large cross-sectional study involving 32,208 patients from 33 countries with T2DM without known albuminuria highlighted the prevalence of microalbuminuria and macroalbuminuria of 39% and 10%, respectively [4]. The prevalence of overt nephropathy was 2.2% and that of microalbuminuria was 26.9% as reported in urban Asian Indians [5]. Recent data highlighted that over 40% of Indian T2DM patients have CKD; interestingly approximately 80% of Indian T2DM patients have good eGFR above 60 ml/min [6]. In view of the increased prevalence of diabetes, patients with DKD will continue to rise. Many patients of DKD will eventually progress to ESRD. Thus, the current challenges are early detection, prevention and improving the DKD outcomes.
Optimum control of blood glucose, blood pressure and lipids along with dietary modifications and smoking cessation will help in halting the progression of DKD. Teneligliptin, a new and relatively economic DPP4 inhibitor is available in India recently [7]. Reported evidence suggests that teneligliptin has a unique place in clinical therapeutics as it can be used in T2DM patients with renal impairment and mild to moderate hepatic impairment [7]. There is a limited data on efficacy and safety of teneligliptin in Asian Indian patients of T2DM with DKD. The objective of this study was to assess efficacy and safety of teneligliptin in Asian Indian patients of T2DM with early DKD at a Diabetes and Thyroid Care Center in Mumbai, India in the real-life scenario.
Materials and Methods
This single center, retrospective study was conducted between April 2016 and August 2016. Data of the patients with T2DM visiting Diabetes and Thyroid Care Center, Mumbai, India from August 2015 to March 2016 were collected and analysed. Data recorded in electronic medical records was extracted and analysed manually. This data included patient demography, past medical history, relevant current medical status, laboratory investigations and medications prescribed.
Following inclusion criteria were applied: patients >18 years of age with T2DM; having early DKD, impaired glycaemic control with existing antidiabetic therapy and received teneligliptin for at least 24 weeks with or without other concurrent glucose lowering therapy, availability of laboratory investigation reports, which included glycaemic parameter, kidney function, and lipid profile. Patients were identified to have DKD if they had decreased eGFR for more than three months, microalbuminuria, and retinopathy [8,9]. Patient having current or previous history of cancers, severe liver disease, ESRD, or a history of gestational diabetes were excluded. The index date was determined as the first date of initiating teneligliptin. All patients received teneligliptin therapy in addition to their existing antidiabetic therapy.
Outcome Measures
Outcome data were noted at baseline visit, after 12 weeks and after 24 weeks. Data related to glycaemic parameters viz., FPG, PPG, HbA1c; kidney function viz., eGFR, serum (Sr.) creatinine, proteinuria and lipid profile were analysed and compared with 12 weeks and 24 weeks. Data for proteinuria with dipstick analysis was available and graded as: trace (15-30 mg/dl); 1+ (30-100 mg/dl); 2+ (100-300 mg/dl); 3+ (300-1000 mg/dl) and 4+ (>1000 mg/dl) [10]. Additionally, data related to retinopathy and neuropathy was analysed. Any reported adverse event or any other recorded safety data was assessed and analysed.
This study was conducted in accordance with the principles of the Declaration of Helsinki, guidelines for good pharmacoepidemiology practice and local regulatory guidelines. Patients who have given their prior consent to use their medical data was collected and analysed in this study.
Statistical Analysis
The study was analysed by using descriptive statistical analysis. Continuous variables were presented as mean±standard deviation and categorical variables were described in frequency and percentage. Efficacy of variables like changes in FPG, PPG and HbA1c were estimated by using Student’s t-test. Other parameters like the change in eGFR, Sr. creatinine, lipid profile were also evaluated by Student’s t-test. Changes in the proportion of cases with proteinuria were compared by Chi-square test. All p-values were reported based on two sided significance test and all the statistical tests were interpreted at 5% level of significance level.
Results
Data of 79 patients was extracted and analysed, out of which 37 patients had early DKD matched to inclusion/exclusion criteria were prescribed teneligliptin 20 mg Once a Day (OD). These patients were analysed for efficacy and safety. Demographic data is presented in [Table/Fig-1]. All patients were instructed for lifestyle modification in terms of dietary modification, physical activity and smoking cessation at the baseline as part of the routine advice. All patients were on Oral Hypoglycaemic Agents (OHAs) and basal insulin; teneligliptin therapy was added to existing antidiabetic therapy to achieve glycaemic control. Antihypertensive and lipid lowering therapy were prescribed.
Baseline patient characteristics.
| Total Number of patients | 37 |
| Age (years) | 62.22±06.77 |
| Sex, n (%) Male Female | 21 (56.8) 16 (43.2) |
| Duration of disease (years) | 13.05±02.44 |
| FPG,(mg/dl) | 143.89±28.26 |
| PPG,(mg/dl) | 200.62±41.88 |
| HbA1c,(%) | 8.65±0.58 |
| eGFR (CKD-EPI),(ml/min/1.73 m2) | 53.35±4.24 |
| Sr. creatinine,(mg/dl) | 2.45±0.27 |
Data expressed as mean±SD. FPG-Fasting Plasma Glucose; PPG-Postprandial Plasma Glucose; HbA1c-Glycated Haemoglobin; eGFR-estimated GFR.
Effect of Teneligliptin on Glycaemic Parameters
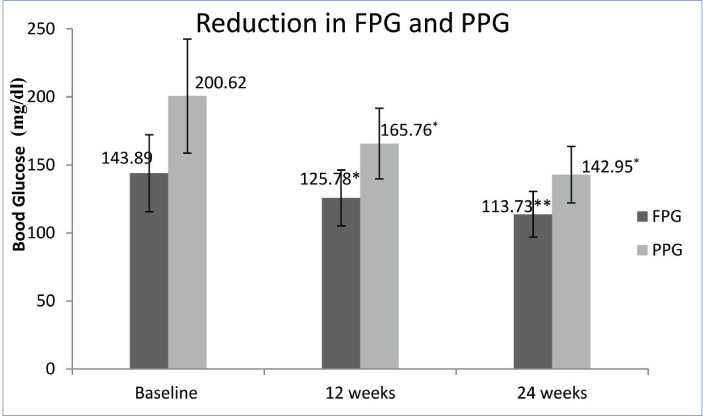
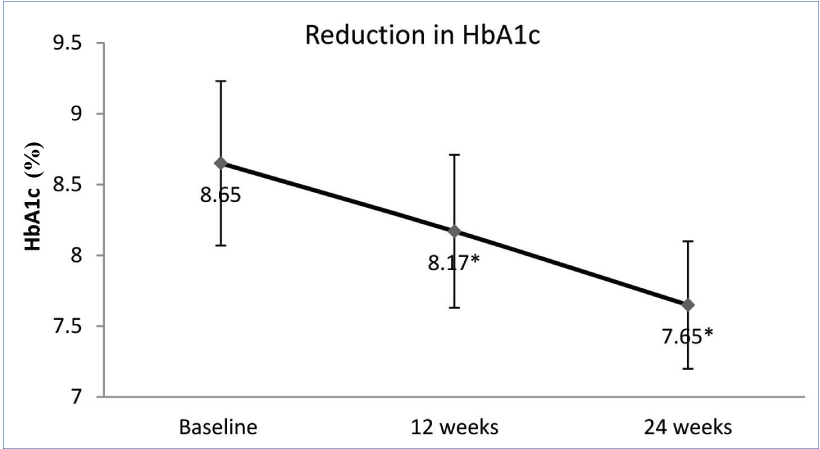
The addition of teneligliptin reported a significant reduction in FPG and PPG at 12 and 24 weeks. Compared to baseline, mean FPG was reduced by 18.11 and 30.16 mg/dl (p=0.001 and 0.008) at 12 and 24 weeks respectively. Similarly mean PPG was reduced by 34.86 and 57.67 mg/dl at 12 and 24 weeks, respectively (p=0.001 for both) [Table/Fig-2]. There was significant reduction of 0.48% (p=0.001) HbA1c at 12 weeks which was sustained up to 24 weeks. At 24 weeks, 1% reduction in HbA1c was noted (p=0.001); [Table/Fig-3].
Reduction in fasting and postprandial glucose over 24 weeks. *p=0.001 when compared with baseline; **p=0.008 when compared with baseline; Student’s t-test
Reduction in HbA1c over 24 weeks. *p=0.001 when compared with baseline; Student’s t-test
Effect of Teneligliptin on Kidney Parameters
Significant improvement in eGFR (CKD-EPI) and Sr. creatinine were noted with the addition of teneligliptin [Table/Fig-4]. At baseline, all patients had eGFR below 60 ml/min/1.73 m2. Within 12 weeks of teneligliptin therapy, significant increase of eGFR was noted (53.35 vs. 55.08 ml/min/1.73 m2; p=0.001) which further improved at 24 weeks (57.95 ml/min/1.73 m2; p=0.001). eGFR was increased by 8.6%. Sr. creatinine was reduced significantly at 12 and 24 weeks. Almost 15% reduction in Sr. creatinine was noted at 24 weeks.
Effect on kidney parameters.
| Baseline | 12 weeks | 24 weeks |
|---|
| eGFR(CKD-EPI),(ml/min/1.73 m2) | 53.35±4.24 | 55.08±4.19* | 57.95±4.38* |
| Serum creatinine,(mg/dl) | 2.45±0.27 | 2.26±0.23* | 2.08±0.22* |
Data expressed as mean±SD.
p=0.001 when compared with baseline; Student’s t-test
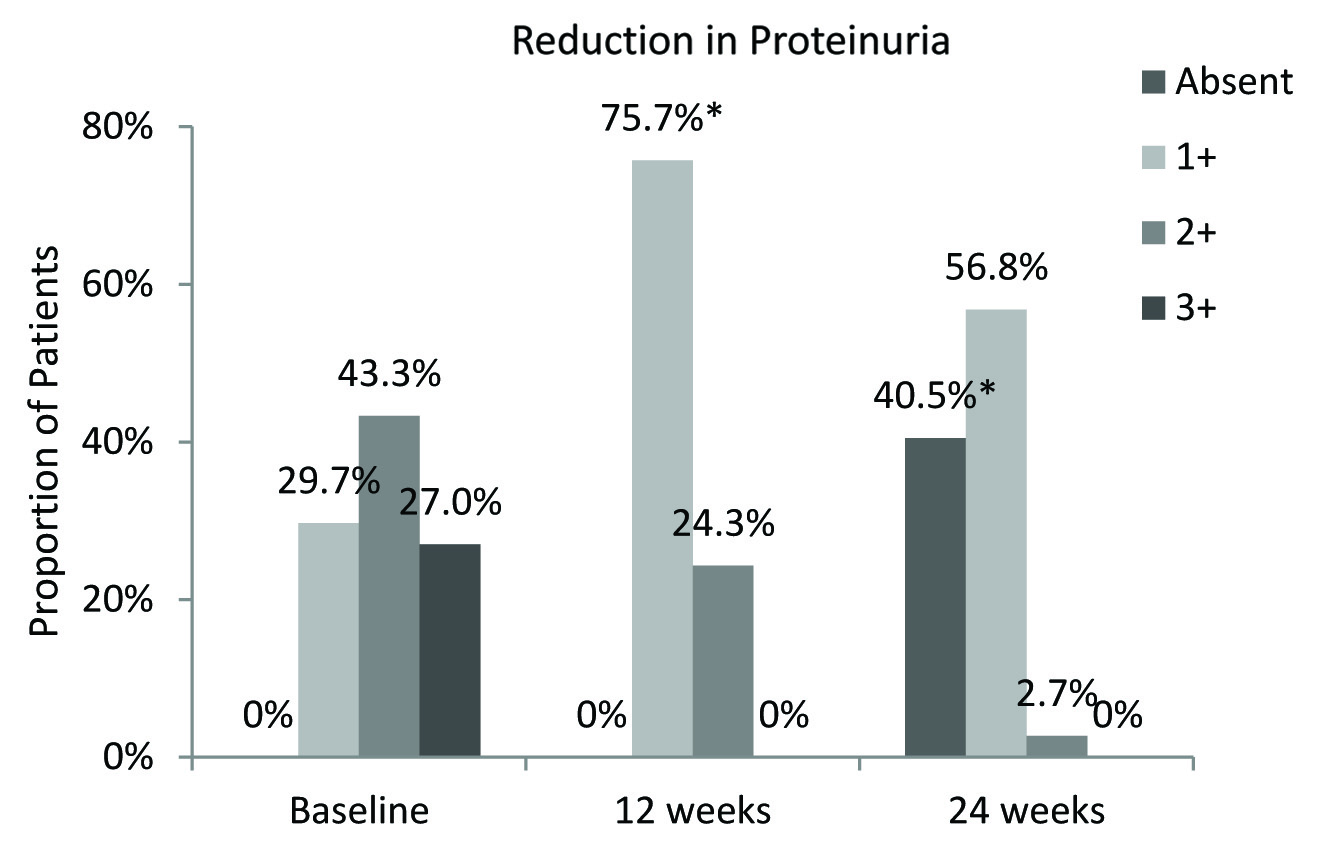
At baseline, all patients had proteinuria; 29.7%, 43.3% and 27% patients reported 1+, 2+ and 3+ Grade proteinuria respectively. At 12 weeks, proteinuria was reduced significantly; 75.7% patient reported Grade 1+ and 24.3% patients reported Grade 2+ and no patient with Grade 3+ proteinuria. At 24 weeks, almost 40.5% patients had no proteinuria and majority patients had Grade 1+ proteinuria [Table/Fig-5]. Significant improvement in lipid variables was also reported during teneligliptin therapy [Table/Fig-6].
Reduction in proportion of patients with proteinuria. *p=0.001; Chi-square Test
Effect on lipid variables.
| Baseline | 12 weeks | 24 weeks |
|---|
| LDL-C, mg/dL | 137.30 ± 37.79 | 118.68 ± 26.37* | 97.05 ± 20.77* |
| HDL, mg/dL | 36.59 ± 4.21 | 39.08 ± 3.54* | 42.43 ± 3.01** |
| TG, mg/dL | 224.78 ± 77.42 | 164.03 ± 40.79* | 122.76 ± 30.26* |
Data expressed as mean±SD.
p=0.001;
p=0.008 when compared with baseline; Student’s t-test
Safety
There was no adverse event recorded in any patient. No hypoglycaemic episode was reported. No cardiovascular event was noted in any patient. All patients had pre-existing Non-Proliferative Diabetic Retinopathy (NPDR). None of the patients reported further deterioration of retinopathy. All patients with pre-existing peripheral neuropathy reported subjective improvement in peripheral neuropathy and quality of life.
Discussion
Pharmacotherapy of T2DM in patients of DKD is a common challenge in clinical practice with regular titration of drugs according to deteriorating renal function. Kidney disease presents a scenario where the chances of hypoglycaemia are increased due to decreased clearance of insulin and reduced renal gluconeogenesis [11]. This is the reason why ADA [12], American Association of Clinical Endocrinologists (AACE) [13] and Kidney Disease Outcomes Quality Initiative (KDOQI) [11] guidelines recommend less stringent HbA1c goals (<7%-KDOQI, <8%-ADA and 7-8%-AACE) for patients of diabetes with CKD.
Evidence suggests that DPP4 inhibitors have many cardiovascular (CV) protective effects beyond glycaemic benefits. Improvement in endothelial function and vasculoprotective effects are postulated for reduction of risk of multiple comorbidities associated with diabetes. Additionally, several beneficial effects on insulin resistance, oxidative stress, dyslipidemia, adipose tissue dysfunction, dysfunctional immunity, and antiapoptotic properties account for CV protection [14].
Signals of antifibrotic and anti-inflammatory activity have been reported with DPP4 inhibitors in kidney disease. DPP4 enzyme is also involved in the advanced glycation end product – receptor axis, which is highly relevant in DKD. DPP4 inhibitors reduce Transforming Growth Factor Beta 1 (TGFβ1), a major driver of fibrosis in DKD [15].
Teneligliptin and linagliptin are the only DPP4 inhibitors which need no dosage adjustment in renal impairment [7,16]. Teneligliptin reduces Platelet-Derived Micro-Particles (PDMPs), P-selectin, E-selectin, Vascular Adhesion Molecule (VCAM-1) and Plasminogen Activator Inhibitor-1 (PAI-1) in patients with T2DM on haemodialysis [17]. PDMPs and PAI-1 are risk factors for cardiovascular disease (CVD).
Published literature highlights clinical importance of teneligliptin in management of glycaemic control in DKD [18]. Teneligliptin is less studied in the Asian Indian diabetic population [19] and even less in those with CKD. Therefore we focused on its effect in patients of early DKD. This was a retrospective study designed to assess the efficacy and tolerability of teneligliptin in patients of T2DM with early DKD in real life scenario. We noticed interesting results for teneligliptin in patients of early DKD.
In the present study, teneligliptin reported significant reduction in FPG and PPG by 30 and 57 mg/dl respectively. At 24 weeks, clinically significant and meaningful 1% reduction in HbA1c was noted. Effects on renal parameters were also found significantly positive in this study, with an increase in eGFR by 8.6%, reduction of Sr. creatinine by 15% and reduction in the proportion of patients with proteinuria. Teneligliptin also improved the lipid parameters with LDL reduction being as much as 29%. These results are very relevant and important in daily clinical practice since renal disease alters the pharmacokinetics of most DPP4 inhibitors. The pharmacokinetics of teneligliptin has a special role in obtaining the results.
The Tmax of teneligliptin is 1 hour and t1/2 is 18.9 hours [7]. Teneligliptin is metabolised and eliminated via both renal and hepatic routes. The major route of excretion of teneligliptin is renal, with approximately 34% of teneligliptin excreted unchanged via the renal route, and 66% being metabolised and eliminated via the hepatic and renal routes [7]. In a study of the pharmacokinetics of a single oral dose of teneligliptin 20 mg, in subjects with renal impairment and ESRD, it was revealed that after single oral dose of teneligliptin 20 mg, Cmax was unaffected by degree of the renal impairment [20]. There was an increase in AUC0–∞ in subjects with renal impairment across the group compared to healthy subjects; however, this was unrelated to the degree of renal impairment. Healthy subjects and subjects with kidney impairment or ESRD tolerated teneligliptin 20 mg well. No dose adjustment in teneligliptin was needed in mild, moderate, or severe renal impairment or ESRD subjects [20]. In the present study, we also noticed that teneligliptin improved the renal parameters in patients of early DKD.
There is evidence of improvement in endothelial function (as measured by reactive hyperaemia index) and reduction in reactive oxygen metabolite levels, specific to teneligliptin [21]. Teneligliptin reduces the Mean Amplitude of Glucose Excursions (MAGE), thus reducing fluctuations [22] and also improves adiponectin levels [17]. These factors are protective against endothelial dysfunction [23]. The antioxidant property can be due to the hydroxyl free radical scavenging capability of teneligliptin [24]. Teneligliptin has been found to lower the urinary levels of Liver-Type Fatty Acid Binding Protein (L-FABP) by around 50% when used for 24 weeks [21]. The L-FABP, found in proximal renal tubules, is a binder of oxidative products of fatty acids and is associated with deteriorating renal function and CV disease in T2DM [25]. It is approved as a new tubulointerstitial biomarker in Japan [25]. These are some probable mechanisms for the positive effects of teneligliptin on the kidney.
The improvement in lipid parameters is possibly due to the reported increase in adiponectin levels [26]. Adiponectin levels negatively correlate with LDL cholesterol and triglyceride values and positively correlate with HDL cholesterol values [27].
No episode of hypoglycaemia was reported in this study. Previous studies also have found low rates of hypoglycaemia with teneligliptin [20,28]. Dose adjustments in patients with renal disease were not needed. There was no worsening of retinopathy or neuropathy with the use of teneligliptin, which indicates that teneligliptin is safe for use in patients of diabetes with multiple comorbidities.
To the best of our knowledge, this is the first report of teneligliptin in Asian Indian patients with DKD in the real-life scenario.
Limitation
The study has some obvious limitation such as retrospective, open label design, small sample size, variation in concomitant antidiabetic therapy, and lack of comparative drug and placebo groups. Also, glycated albumin levels and C-peptide levels could not be retrieved. Background therapy for diabetes, hypertension and lipid control is not included in present analysis, which will be updated in upcoming publication. Additionally, it is difficult to prove that improvement of kidney function was due to teneligliptin or improvement in glycaemic parameters. Considering these limitation, results of this study must be interpreted with caution and cannot be generalised. A well designed, large sampled RCT is required to estimate the place of teneligliptin in Asian Indian patients with early DKD.
Conclusion
This 24 week, retrospective study in real life scenario, reported significant improvement in glycaemic control with teneligliptin in Asian Indian patients of T2DM with early DKD with favourable tolerability. Additionally, teneligliptin reported improvement in eGFR and serum creatinine. Thus teneligliptin has an important place in clinical therapeutics for the management of T2DM patients with renal impairment.
Data expressed as mean±SD. FPG-Fasting Plasma Glucose; PPG-Postprandial Plasma Glucose; HbA1c-Glycated Haemoglobin; eGFR-estimated GFR.