Infiltrating Ductal Carcinoma Co-Existing with Intraductal Papillary Carcinoma of Male Breast: A Rare Case Report
Mayank Kumar1, Bhaswanth Pottipati2, Surekha U. Arakeri3, Anita P. Javalgi4
1 Postgraduate Student, Department of Pathology, BLDE University’s Shri B.M. Patil Medical College, Vijayapur, Karnataka, India.
2 Postgraduate Student, Department of Pathology, BLDE University’s Shri B.M. Patil Medical College, Vijayapur, Karnataka, India.
3 Professor, Department of Pathology, BLDE University’s Shri B.M. Patil Medical College, Vijayapur, Karnataka, India.
4 Assistant Professor, Department of Pathology, BLDE University’s Shri B.M. Patil Medical College, Vijayapur, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mayank Kumar, Department of Pathology, Shri B.M. Patil Medical College, Sholapur Road, Vijayapur-586103, Karnataka, India.
E-mail: mayankkumar1618@gmail.com
Male breast carcinomas are rare tumours, accounting for less than 1% of all malignancies in men. Intracystic Papillary Carcinoma (IPC) in males is a very rare entity, representing 5-7.5% of all male breast carcinomas. It lacks the classical clinical, radiological and cytological features of malignancy and usually presents as a benign-appearing lump. We report a case of Infiltrating Ductal Carcinoma (IDC) co-existing with intracystic papillary carcinoma in a 53-year-old male who presented with lump in the right breast.
Lump, Malignancy, Papillary lesion, Tumour
Case Report
A 53-year-old male presented with a single mass in the right breast for three months. It was painless, did not increase in size, and was not associated with nipple discharge. There was no history of any obvious preceding trauma. It was firm, mobile, non-tender and without involvement of the overlying skin or nipple. There were no palpable lymph nodes in the axillary region. The contralateral breast was unremarkable on examination.
Routine haematological and biochemical investigations were within normal limits, with a total leukocyte count of 7,200/cumm and haemoglobin level of 12.4 g/dl. The Fine Needle Aspiration Cytology (FNAC) smears showed moderate cellularity with pleomorphic cells having round to oval vesicular nuclei with prominent nucleoli and moderate amount of cytoplasm. A diagnosis of IDC was made following which a Modified Radical Mastectomy (MRM) of the right breast was performed. The resected specimen was received for histopathological examination.
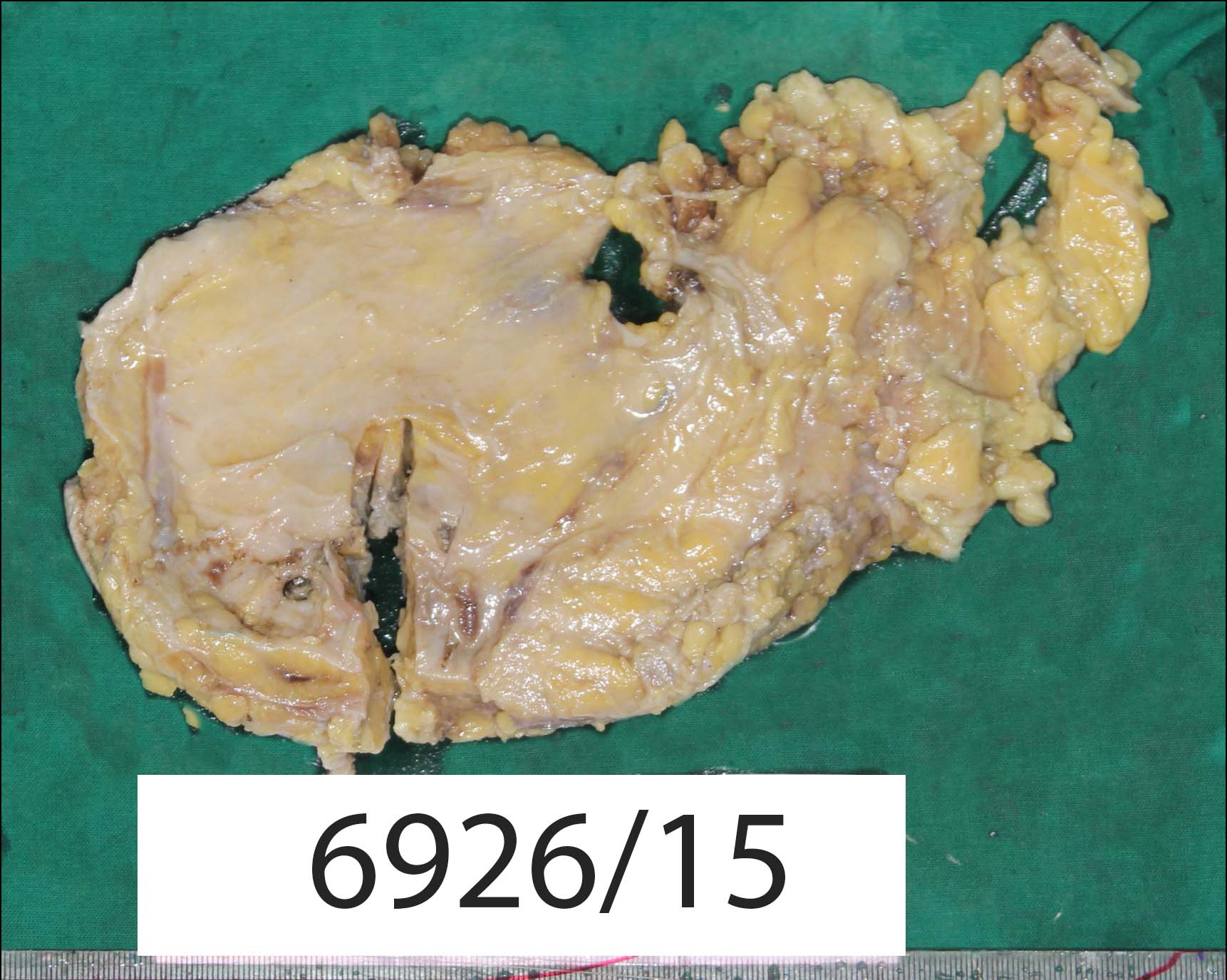
Grossly, the mastectomy specimen measured 26 x 15 x 3.5 cm [Table/Fig-1]. On serial sectioning, a firm mass was noted in the sub areolar region, measuring 4 x 3.5 x 2 cm. The cut surface was pale pink in colour and showed a partly solid and partly cystic mass with blood mixed mucoid material within the cystic areas. Six lymph nodes were retrieved from the attached axillary fat.
Gross photograph of modified mastectomy specimen.
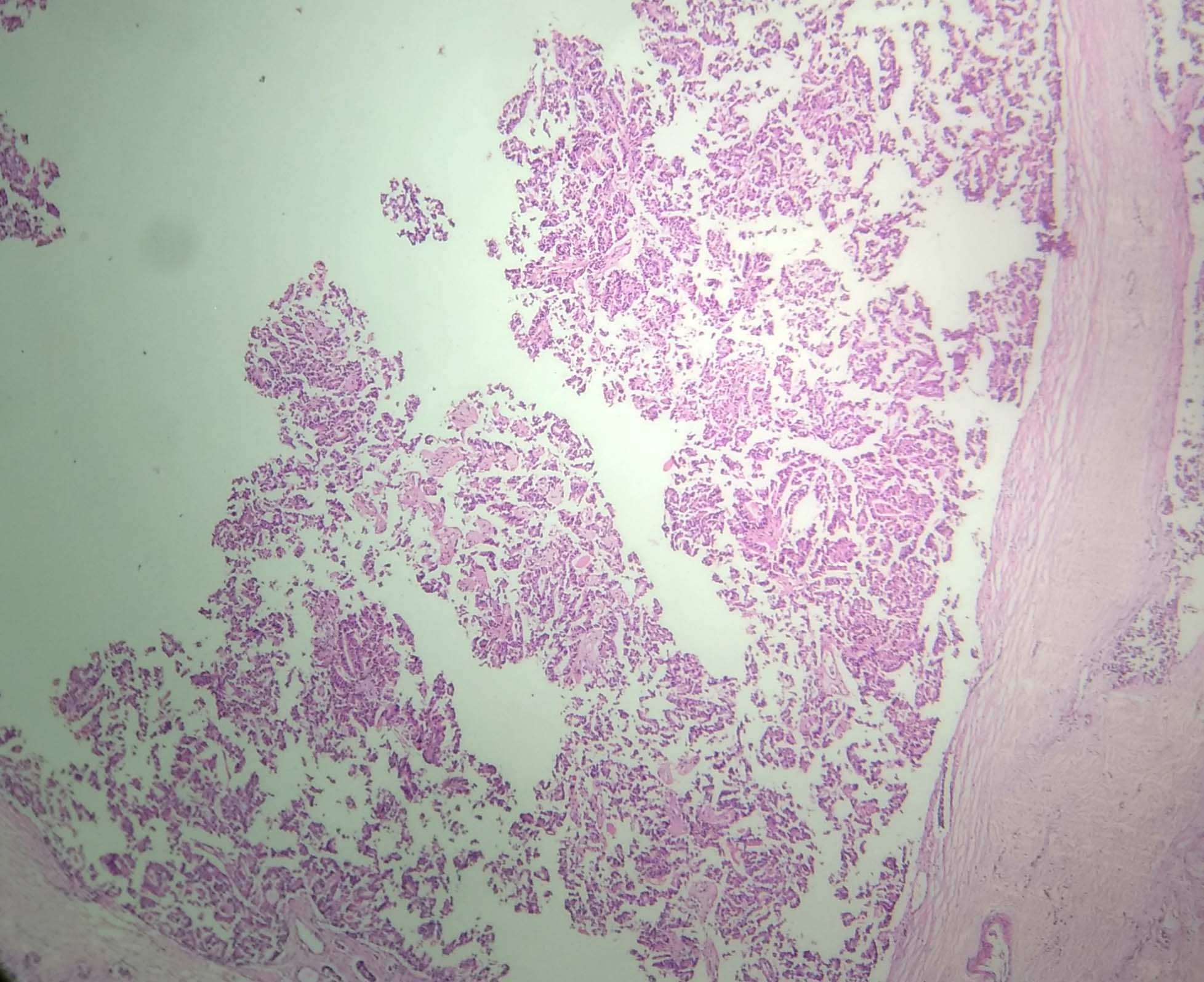
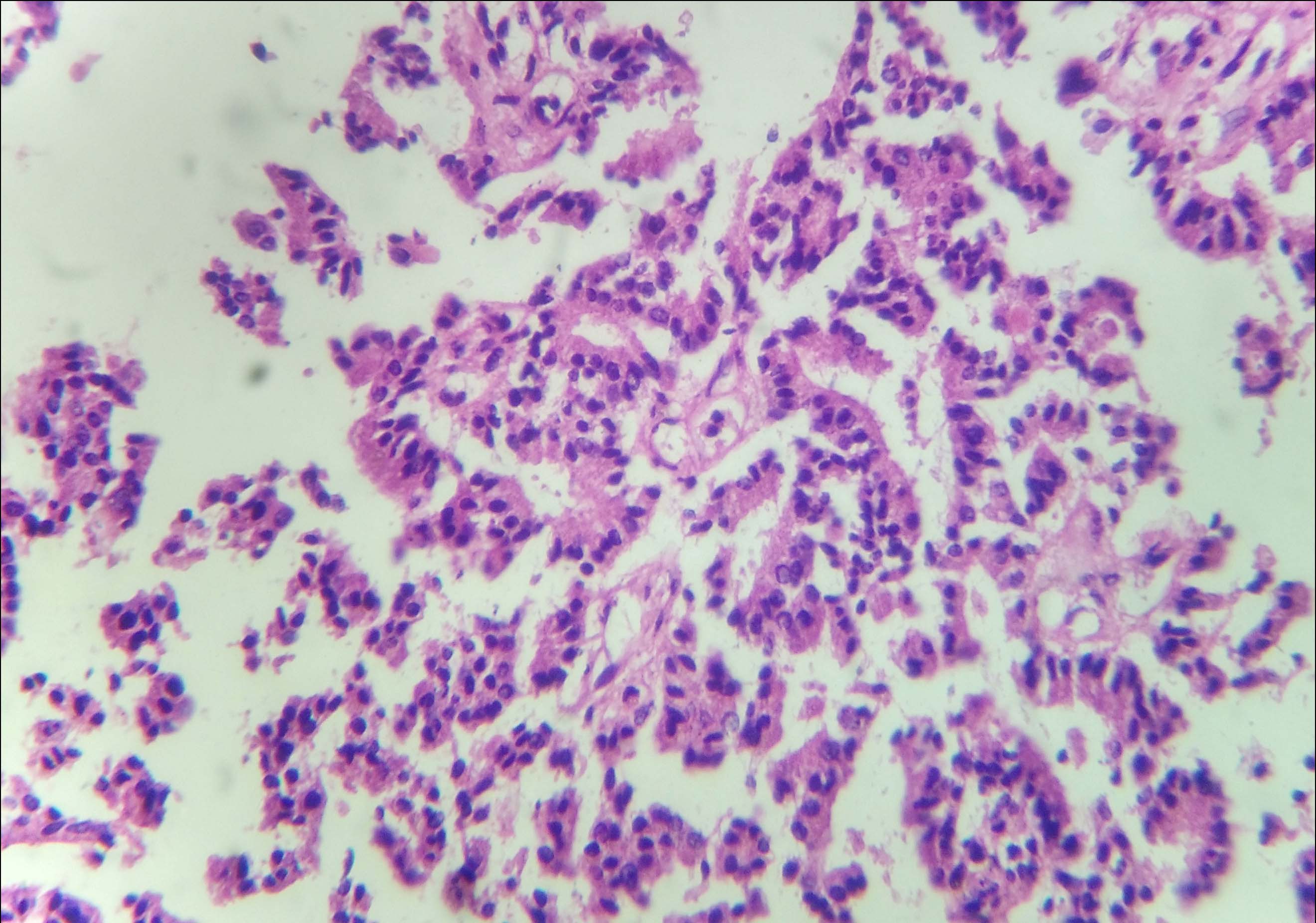
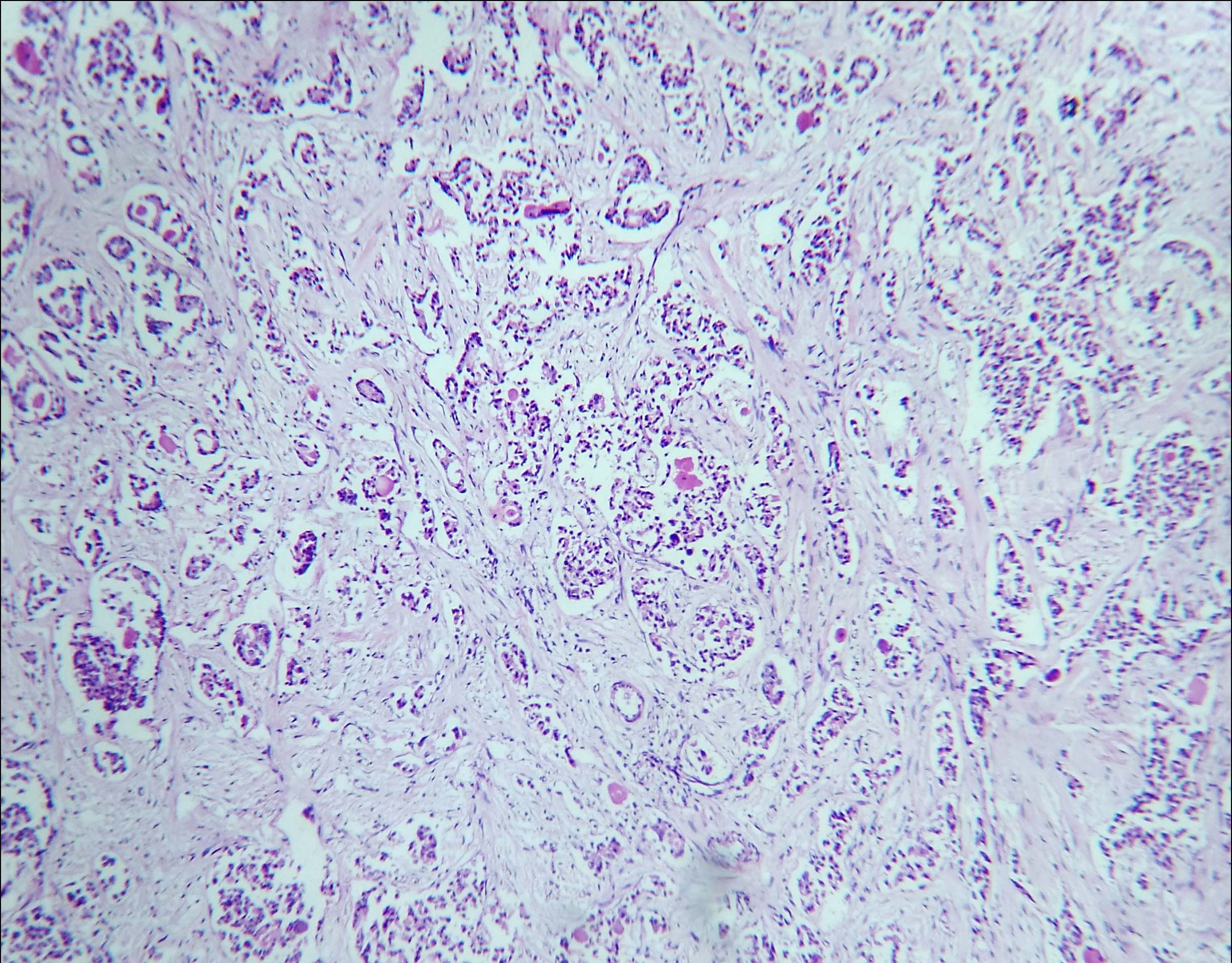
On microscopic examination, tumour tissue was arranged in micro-papillary, micro-cystic patterns and solid nests which were separated by fibro-collagenous septa. Few cystically dilated ducts were seen, with micro-papillary arrangement of tumour cells. These tumour cells were columnar to polygonal, having round to oval hyperchromatic nuclei and variable amount of eosinophilic cytoplasm [Table/Fig-2,3]. In other areas, tumour tissue was arranged in tubular and acinar patterns [Table/Fig-4]. The tumour cells in these areas had vesicular nuclei with prominent nucleoli and irregular nuclear borders. Comedo necrosis was seen at foci. Axillary lymph nodes were free of tumour tissue.
A cystically dilated duct with micro-papillary arrangement of tumour tissue (H&E, 4X).
Tumour cells arranged around fibro-vascular core were polygonal to columnar, having round to oval hyperchromatic nuclei and variable amount of eosinophilic cytoplasm (H&E, 10X).
Other areas showed tumour tissue arranged in tubular and acinar patterns (H&E, 4X).
Based on the microscopic findings, a diagnosis of IDC – Not Otherwise Specified (NOS) with associated IPC was made.
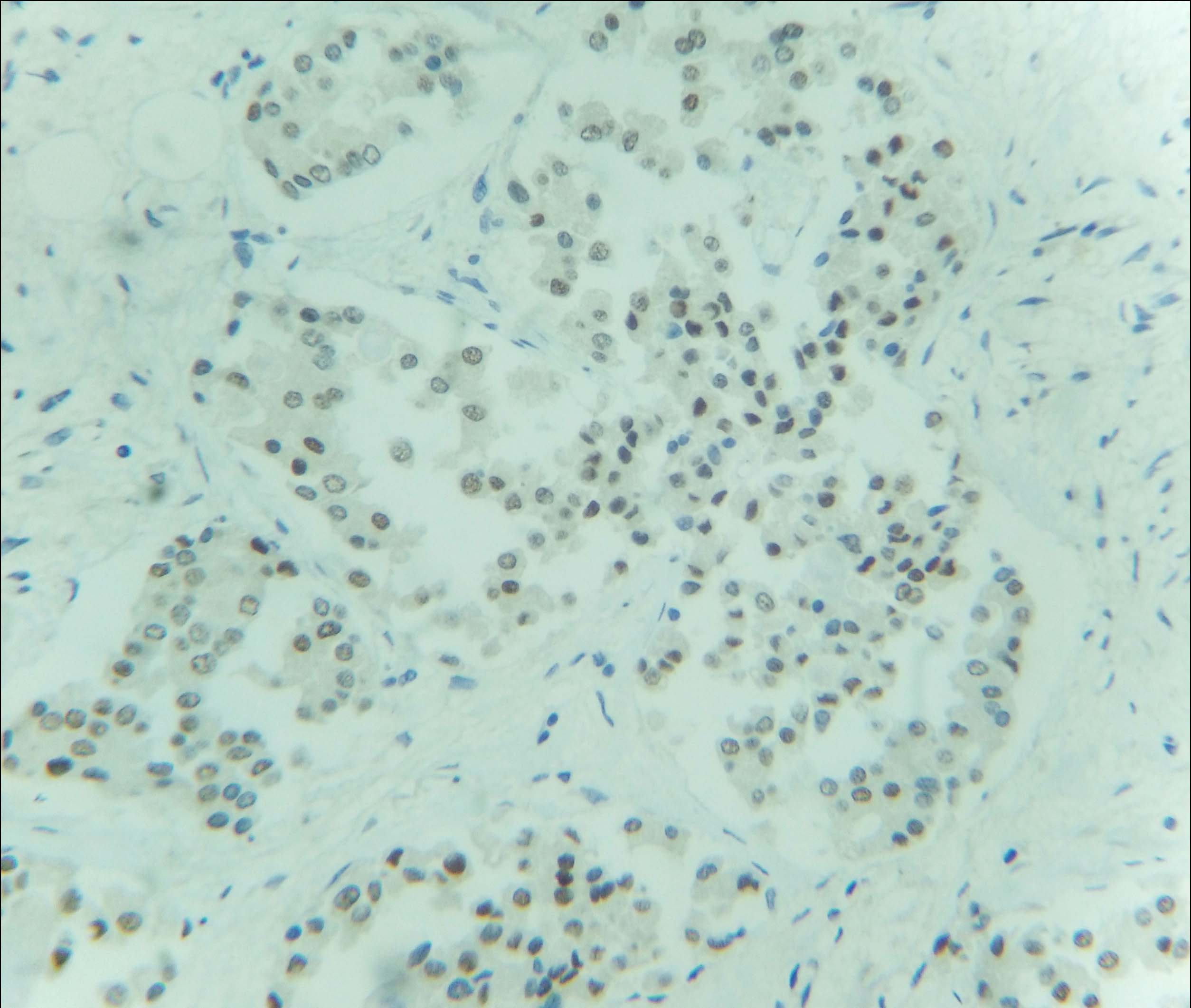
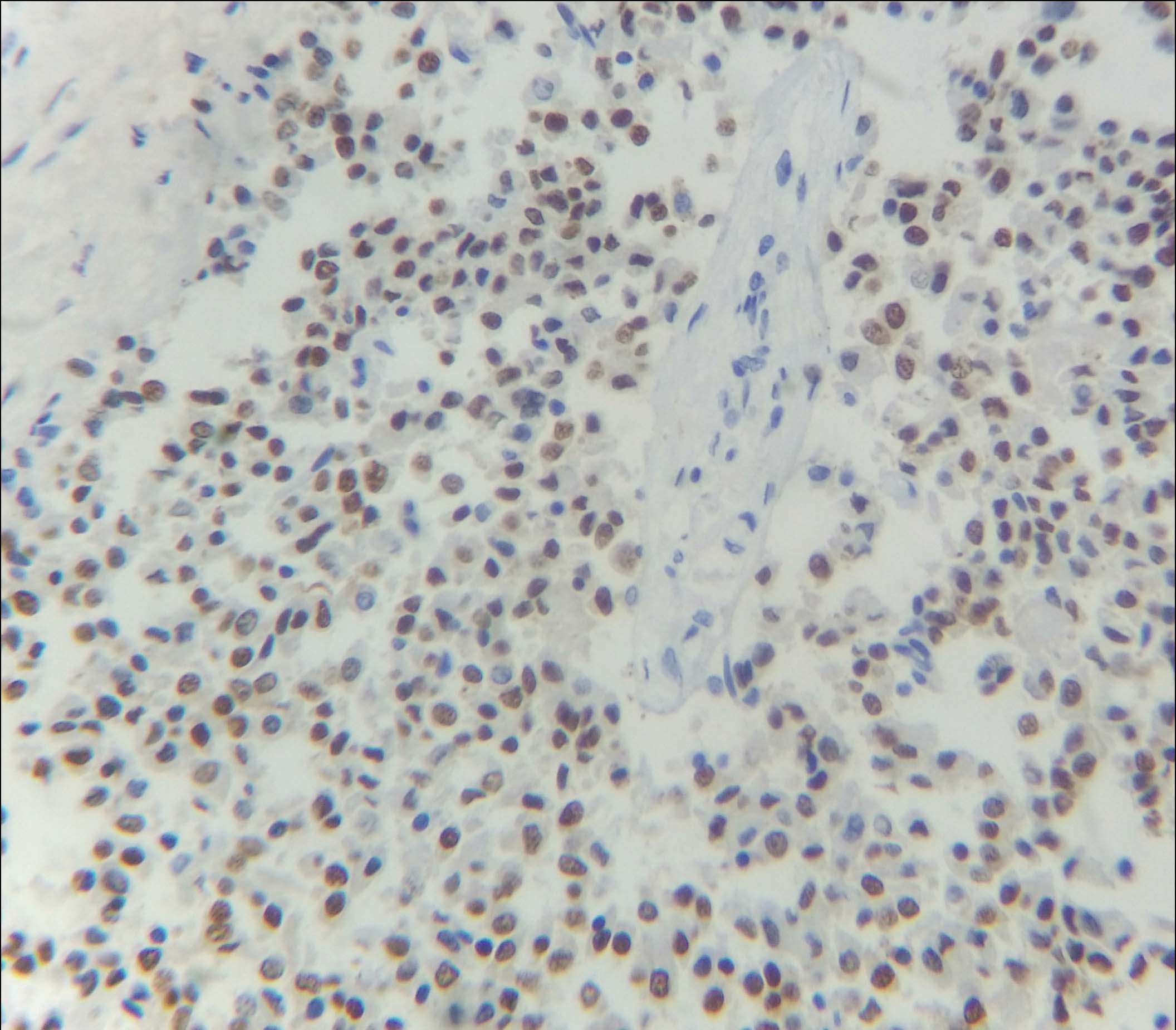
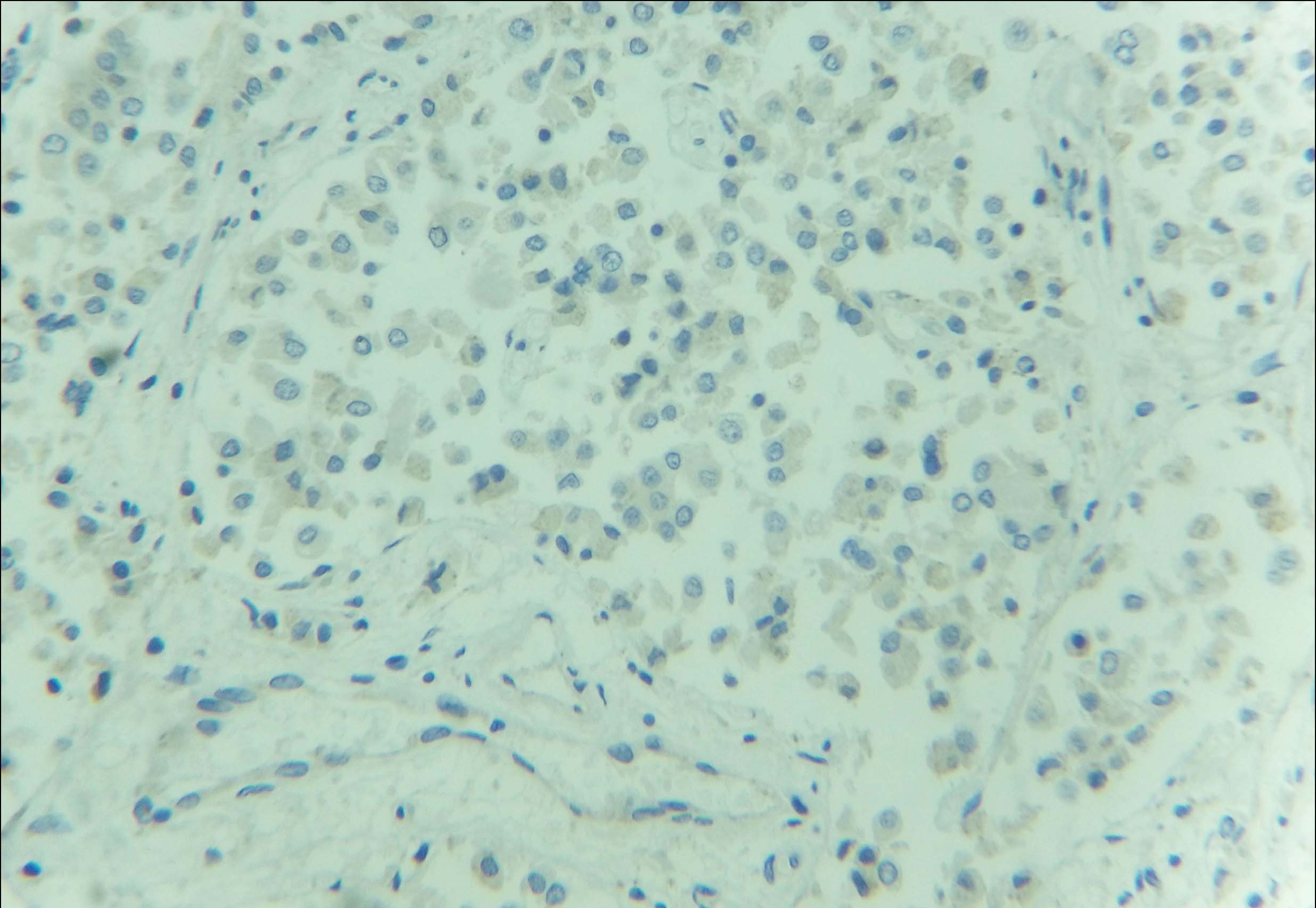
Immunohistochemical examination (IHC) was subsequently performed. The tumour cells were positive for Oestrogen (ER), Progesterone (PR) and negative for Her2neu receptor [Table/Fig-5,6 and 7]. The scores for proportion were 5 and 4 for ER and PR receptors respectively, as per the Allred scoring system. Majority of the cells showed moderate to strong nuclear staining for these two receptors. One year follow up in the patient revealed no evidence of recurrence or metastasis.
IHC showed nuclear positivity for oestrogen receptor (40X).
IHC showed nuclear positivity for progesterone receptor (40X).
IHC showed negativity for Her-2/neu receptor (40X).
Discussion
Male breast carcinoma is an uncommon condition, accounting for less than 1% of all malignancies in men. IPC in males is a very rare entity, representing only 5-7.5% of all male breast carcinomas [1].
IPC is usually seen in post-menopausal women, more commonly above 55 years of age. It presents clinically as a benign-appearing, well-circumscribed, mobile mass in the breast, without involvement of the nipple or overlying skin [1,2].
An accurate preoperative diagnosis of IPC of the male breast requires careful assessment of clinical, radiological and cytological findings. IPC usually appears as a well-defined density on mammography. Ultrasonography (USG) typically shows a hypoechoic area with soft tissue echoes projecting from the wall of the cyst [2]. The differential diagnoses on radiology include a spectrum of benign to malignant lesions, such as haematoma, benign cyst, adenofibroma, IDC, colloid or medullary carcinoma [3].
In pure IPC, FNAC frequently gives false negative results due to the underlying cystic nature of the lesion [4]. However, in the present case, a diagnosis of IDC was offered on FNAC due to the presence of an associated invasive component. Due to the non-specific radiological and cytological findings in pure IPC, excisional biopsy is usually necessary to establish a diagnosis of IPC.
Papillary carcinomas of the breast are characterized by papillary growth pattern, having a fibro-vascular stalk that is lined by neoplastic epithelial cells. IPC is a malignant papillary proliferation within a cystically dilated duct [5].
Histopathologically, IPC can be of three subtypes – pure IPC, IPC associated with ductal carcinoma in situ, and IPC associated with invasive carcinoma [2]. The occurrence of an associated lesion has an effect on the treatment protocol. This case was associated with an invasive component.
IPCs usually show strong immunopositivity for ER, PR and negativity for Her2neu, which was seen in this case [5].
The aetiology of IPC is unknown. It is more commonly seen in the larger, centrally placed ducts and it is thought that cystic dilatation of the duct occurs due to tumour development and its secretion. The malignant nature of such lesions can be confirmed by determining the Loss of Heterozygosity (LOH) on chromosome 16q, which is not seen in intraductal papillomas [6].
Axillary lymph node metastasis occurs in 14% cases [7]. Our case did not show any metastasis in the retrieved axillary lymph nodes. No definite guidelines are available regarding the management of IPC. This is due to very low incidence and incomplete histopathological classification. Surgery is the preferred modality of treatment, conservative resection or MRM depends on the association, if present, with Ductal Carcinoma In Situ (DCIS) or invasive carcinoma [8]. In the present case MRM was done as IPC was associated with invasive carcinoma. There was no evidence of recurrence or metastasis on one year follow up.
Conclusion
IPC in males is a rare malignancy of the breast, having favourable prognosis in its pure form. However, if IPC is associated with IDC, surgical resection with adjuvant therapy is must. Hence its presence should be suspected when an intracystic nodule is seen on USG. Given the non-specific clinical and radiological features, clinical suspicion and thorough histopathological examination of the resected lesion are necessary for an accurate diagnosis of associated IDC in IPC.
[1]. Sinha S, Hughes RG, Ryley NG, Papillary carcinoma in a male breast cyst: A Diagnostic ChallengeAnn R Coll Surg Eng 2006 44:88-91. [Google Scholar]
[2]. Muallaoglu S, Ozdemir E, Kutluay L, Intracystic papillary carcinoma of the breast in a male patient: a case reportCase Rep Med 2012 2012:10-14. [Google Scholar]
[3]. Kumar H, Ail DA, Prakash G, Intracystic papillary carcinoma of the breast with associated invasive carcinoma: a rare case reportRes Rev J Med Heal Sci 2014 3:43-46. [Google Scholar]
[4]. Romics L, Brien MEO, Relihan N, Connell FO, Redmond HP, Intracystic papillary carcinoma in a male as a rare presentation of breast cancer: A Case Report and Literature ReviewJ Med Case Rep 2009 4:2-5. [Google Scholar]
[5]. Kishor HS, Dhiraj BN, Rajshri PD, Dravid N, Tayde Y, An Unusual case of intracystic papillary carcinoma of breast with invasive componentInt J Med Res Heal Sci 2014 3:762-65. [Google Scholar]
[6]. Imoto S, Hasebe T, Intracystic Papillary Carcinoma of the Breast in Male: Case Report and Review of the Japanese LiteratureJpn J Clin Oncol 1998 28:517-20. [Google Scholar]
[7]. Shukla S, Singh S, Pujani M, Intracystic papillary carcinoma in a male breast following mastectomy for infiltrating ductal carcinomaIndian J Cancer 2010 47:349-51. [Google Scholar]
[8]. Stamatakos M, Stefanaki C, Stasinou T, Papantoni E, Alexiou I, Kontzoglou K, Breast care intracystic papillary carcinoma of the breast in males. in search of the optimal treatment for this rare diseaseBreast Care 2011 6:399-403. [Google Scholar]