Background
For over half a century, clinicians and pathologists have recognized a group of pulmonary diseases associated with an accumulation of lymphoid follicles in the walls of bronchi and bronchioles. This entity was first described in patients with bronchiectasis, a disease characterized by extensive airway mural inflammation, where lymphocytic hyperplasia in the walls of small airways formed part of the inflammatory picture. However, in a group of patients, pathologists were able to confirm that sub-epithelial accumulation of lymphoid follicles was the primary pathology affecting the bronchioles [1]. These enlarged follicles would often distort the architecture of the bronchial tree, merely due to their size, projecting into the bronchial lumen and causing partial bronchial and bronchiolar obstruction.
In 1947, the first reference to this entity was made by Engel et al., who coined the term “nodal bronchiolitis”, describing thickened bronchioles, with well formed lymphoid follicles in their walls in the absence of a diffuse inflammatory infiltrate [2].
In 1952, Whitewall studied 200 consecutive lung specimens mainly from lobectomies and pneumonectomies of patients with advanced bronchiectasis. He described this feature as “follicular bronchiectasis”, where the most prominent microscopic finding was an extensive formation of lymphoid follicles and lymph nodes in the walls of affected bronchi and bronchioles [1].
In 1979, Epler et al., described an association between bronchiolitis and the administration of D-penicillamine in 2 patients with rheumatoid arthritis and eosinophilic fasciitis. The chronic inflammatory bronchiolar disorder seen was characterized by extensive proliferation of lymphoid tissue, occurring as follicles in the bronchiolar walls and was given the name "follicular bronchiolitis" (FB) [3].
Currently, FB is classified as one of the non-neoplastic (reactive) pulmonary lymphoid disorders in a group known as the lymphoproliferative pulmonary diseases (LPDs) [Table/Fig-1]. It is mainly distinguished from other reactive pulmonary lymphoid diseases in its group such as lymphocytic interstitial pneumonia (LIP) and nodular lymphoid hyperplasia (NLH) by the pattern and extent of pulmonary parenchymal involvement.
Classification of lymphoproliferative pulmonary diseases
| Lymphoproliferative Pulmonary Diseases (LPDs) |
|---|
Reactive/non-neoplastic lymphoid lesions: classified based on the pattern of pulmonary involvementNodular lymphoid hyperplasia (NLH): focal Follicular bronchiolitis (FB): peribronchial Lymphoid interstitial pneumonia (LIP): diffuse with pulmonary cyst
|
Malignant parenchymal lymphoproliferative lesionsPrimary (0.5% of all primary lung neoplasms)Extranodal marginal zone lymphoma of MALT origin (MALT lymphoma) Diffuse large B-cell lymphoma (DLBCL) lymphomatoid granulomatosis (LYG)
|
SecondaryNon-Hodgkin lymphoma (NHL) Hodgkin lymphoma (HL)
|
Lymphoproliferative disorders in the immunocompromisedAcquired immune deficiency syndrome (AIDS)- related lymphoma (ARL) Post-transplantation lymphoproliferative disorder (PTLD)
|
Pathophysiology
Bronchioles are small airway divisions arising from the tertiary bronchi with an average internal diameter of 1mm, where the airway becomes devoid of hyaline cartilage. The term bronchiolitis refers to inflammation of the bronchioles with considerable sparing of the larger airways and lung parenchyma. FB is characterized by the presence of hyperplastic lymphoid follicles that are prominent and well-defined reactive germinal centers distributed along bronchovascular bundles and associated with minimal interstitial disease [3].
Bronchus-associated lymphoid tissue (BALT) is a dense cluster of lymphocytes with follicular structures commonly distributed in a reticular network of stromal cells associated with specialized airway epithelium. BALT is characterized by three main features; 1) Development of a stromal cell network with separation of B and T cell areas; 2) Aggregation of follicular dendritic cells (FDCs) in the B cell follicles; 3) Development of high endothelial venules (HEVs) and lymphatics in the follicular structures [4].
In 1973, the term “BALT” was introduced by Bienenstock et al., as they described the morphologic and functional characteristics of lymphoid aggregates in the respiratory tract of rabbits [5]. In rabbits, BALT is constitutively present in the lungs, seen along the bronchial tree, interlobular septa and within the subpleural nodes. This structure is comparable to the gastric mucosal associated lymphoid tissue (MALT) and intestinal associated lymphoid tissue (Peyer’s patches).
Studies in humans, however, show that BALT does not exist constitutively as a normal structural component of the airways, but rather an ectopic lymphoid tissue that develops in the lungs in response to antigenic stimulation through inflammation or infection, in a process known as “lymphoid-neogenesis” [6,7]. Once formed, BALT seems to persist in the lungs for up to 3 months after clearance of an infection. During pulmonary inflammation, the perivascular spaces become densely packed with lymphocytes, a process referred to as “perivascular cuffing”. Since pulmonary arteries run parallel to the small airways, lymphocytic inflammation may extend into the airways forming “Induced-BALT” [8].
Induced BALT collects airway antigens and primes naive B cells and T cells. These airway germinal centers can generate memory T cells and plasma cells that remain in the airway and bone marrow, where they respond locally to a secondary challenge, promoting a rapid and efficient immune response to pulmonary pathogens [9].
BALT can be seen in association with many chronic pulmonary diseases, such as asthma, COPD, malignancy, rheumatoid lung disease, and tuberculosis [10–12].
Classification
FB can be generally classified based on its underlying aetiology into a primary and secondary form.
Secondary follicular bronchiolitis is a relatively common disease that occurs in association with many systemic and pulmonary diseases summarized in [Table/Fig-2]. Idiopathic (Primary) form of follicular bronchiolitis is a rare disease that occurs without an associated primary immunodeficiency state, inflammatory, autoimmune, infectious, or connective tissue disease.
Diseases associated with follicular bronchiolitis
| Connective tissue disease |
| Sjögren’s syndrome [13,14]Rheumatoid arthritis [15,16]Systemic lupus erythematosis [17,18] |
| Other immunological disorders |
| Evans Syndrome (Autoimmune haemolytic anaemia and immune thrombocytopenia) [19,20]Pernicious anaemia [21] |
| Immunodeficiency |
| AIDS, particularly in children [22]Common variable immunodeficiency (CVID) [23,24] |
| Infections |
| Pneumocystis Jirovicci pneumonia [25]Legionella pneumonia [26]Active hepatitis [27] |
| Interstitial lung diseases [24, 28–30] |
| LIPRespiratory bronchiolitis-ILD (RB-ILD)Desquamative interstitial pneumonia (DIP)Hypersensitivity pneumonitis (HP)Cryptogenic organizing pneumonia (COP)Granulomatous lymphocytic-ILD (GLILD) |
| Airway inflammatory diseases [10,11] |
| BronchiectasisAsthmaCOPD |
| Familial [31,32] |
| Idiopathic (primary) |
Clinical Presentation
Secondary follicular bronchiolitis may occur at any age, while the idiopathic form is most commonly seen in middle-aged and elderly patients.
FB can be generally classified into three clinicopathalogical groups based on the patients’ clinical presentation and the presence or absence of underlying systemic diseases [16,33,34]: Group 1 includes patients with an underlying connective tissue disease, most commonly rheumatoid arthritis (RA) and Sjögren’s syndrome (SS), who often present in their 5th decade with progressively worsening dyspnea as the main symptom in almost all cases and in 88% of patients the diagnosis of connective tissue disease proceeds the respiratory manifestations.
Group 2 includes patients with an immunodeficiency, either congenital or acquired, e.g. Common variable immune deficiency (CVID) and acquired immunodeficiency syndrome (AIDS), respectively, where the clinical presentation is that of a teenager or young adult with recurrent pneumonias and progressive dyspnea.
Group 3 constitutes a heterogeneous group of patients, middle age and elderly, who have no known immunodeficiency and no clinical or serological evidence of a connective tissue disease, often presenting with a chronic cough and peripheral eosinophilia, suggesting an underlying hypersensitivity reaction. This group has also been referred to as “idiopathic (primary) follicular bronchiolitis” [35,36].
Pulmonary function testing in patients with FB is often nonspecific, and can reveal a normal, restrictive, obstructive or mixed pattern of airflow limitation.
Radiological Findings
Chest radiography is often normal in primary FB, but may reveal lung hyperinflation due to air trapping, small nodules, reticular, or reticulonodular infiltrates in a severe bronchiolar disease [37]. The most common features of FB on high resolution computed tomography (HRCT) scan are small centrilobular nodules (1–3 mm) in diameter often associated with bilateral patchy ground-glass opacities [38].
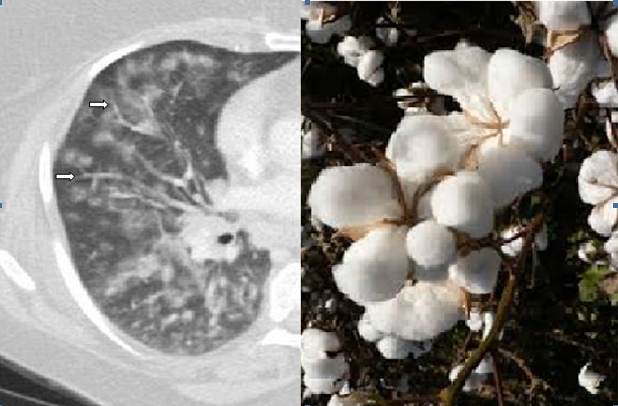
One of the most common distinctive signs of bronchiolar disease on HRCT is a “Tree-in-bud” pattern, representing bronchiolar impaction with exudative material secondary to infection or inflammation [39] [Table/Fig-3]. The presence of peribronchial inflammation and peribronchial lymphoid follicle formations can give a unique fluffy appearance to the tree-in-bud on HRCT, as the lymphoid follicles become densely concentrated in the interstitium adjacent to the bronchioles and fade away from the interstitium furthest from the airway. We here in describe this unique pattern which may assist in differentiating follicular bronchiolitis from other types of infectious or inflammatory bronchiolitis on HRCT as a “cotton-in-bud” appearance [Table/Fig-4].
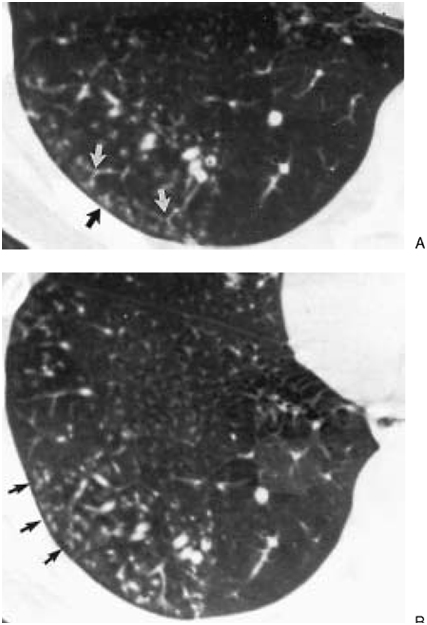
Infectious bronchiolitis with a tree-in-bud appearance and small well-defined centrilobular nodules (arrows). These reflect the presence of exudate-filled centrilobular bronchioles
In Follicular bronchiolitis, dilated and thick-walled bronchioles (white arrows) are seen in association with a fluffy tree-in-bud “Cotton-in-bud” appearance. These findings correlate pathologically with bronchiolar follicular obstruction and peribronchiolar interstitial lymphoid follicles, respectively
Air trapping due to bronchiolar obstruction can also produce a mosaic pattern of lung attenuation (geographic areas of variable densities) often depicted on expiratory HRCT imaging. [Table/Fig-5] illustrates the main features of FB on HRCT scan.
Cardinal HRCT features of follicular bronchiolitis
| Main CT features of FB |
|---|
Bilateral 1–3 mm nodules—centrilobular/peribronchial distribution Bilateral patchy ground-glass opacities (mosaic pattern) Fluffy tree-in-bud “Cotton-in-bud” peribronchiolar opacities Bronchial dilatation Disease is limited to the airways (i.e. no diffuse interstitial involvement)
|
Histopathology
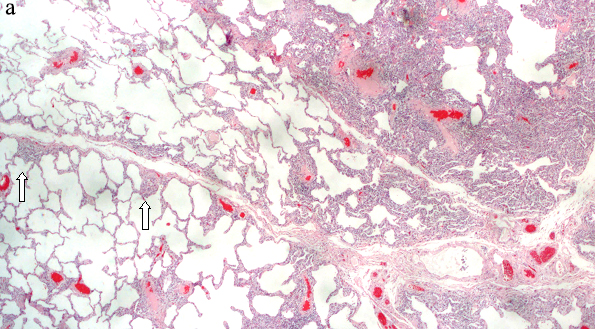
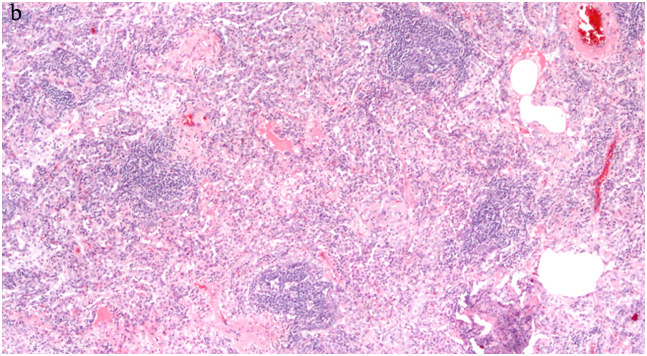
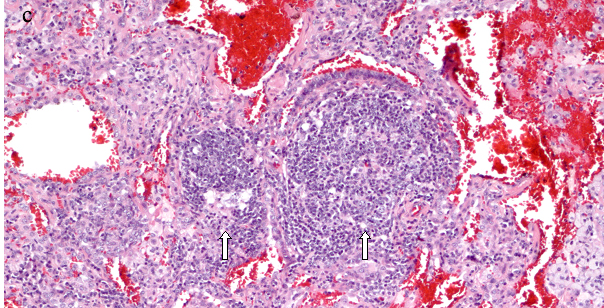
Diagnosing FB on pathological specimens requires demonstrating two fundamental features, first, the presence of well formed lymphoid follicles in the walls of bronchioles and the second is narrowing or complete obliteration of the bronchiolar lumen [Table/Fig-6a-c]. In these pathological specimens the main distinguishing feature between FB and LIP is the extension of lymphoid follicles along the interlobular septa with substantial sparing of the alveolar septa in FB, where as extensive alveolar septal involvement is considered the hallmark of LIP [23,27,34]. Nonetheless, many pathologists today describe these two entities as a continuum of reactive pulmonary lymphoid diseases, in which the distinction in many cases is somewhat arbitrary. Secondary features that can be associated with FB on pathologic specimens include foci of organizing pneumonia, foci of obstructive pneumonia and bronchiolar intraluminal neutrophilic infiltrate [40].
Surgical lung biopsy (a) HE, X100 shows lymphocytic infiltrates in germinal centers in a peribronchial distribution and extending into the interlobular septa (arrows) sparing the alveolar septa. (b) Higher power view HE, X 200 The peribronchial lymphoid tissue has germinal center, and consists of mature lymphocytes, (c) A large germinal center seen adjacent to the bronchiole (right arrow), another germinal center involving the bronchial wall and projecting into the bronchial lumen, almost completely occluding the bronchiole (left arrow) HE, X400
Immunohistochemistry in FB is essential to rule out malignancy. In the absence of a primary immunodeficiency state, FB lesions often reveal CD20 and CD79a positive B cells predominantly within peribronchial lymphoid aggregates and CD3 positive T cells predominantly in the alveolar interstitium when overlapping with LIP [41,42].
Differential Diagnosis
A pathologic diagnosis of FB is aetiologically non-specific, as it may represent a primary condition, or a secondary pathological finding associated with other pulmonary or systemic diseases. Hence, a clinical diagnosis after establishing FB on pathological evaluation requires correlating the pathology with clinical and radiographic findings.
The main differential process from a clinical standpoint is a stepwise approach that entails identifying the distinctive features of FB from other closely related diseases in the LPDs group, this step requires recognizing the unique patterns of bronchiolar and pulmonary involvement on HRCT and close microscopic evaluation of airway and parenchymal follicular extensions, along with immunohistochemistry staining to rule out malignancy. Next step is differentiating between primary and secondary FB by recognizing other clinical features or multiple organ involvement, with the aid of serological and immunodeficiency testing.
Treatment and Prognosis
In secondary FB, management is usually aimed at treating the underlying condition. FB associated with HIV has been shown to improve with the initiation of anti-retroviral therapy [43], and when associated with a connective tissue disease, FB is generally approached with the same treatment modalities of the primary disease, which often involves the use of immunosuppressant therapy.
In FB with underlying CVID, intravenous immunoglobulin replacement therapy can significantly reduce the frequency and severity of pulmonary infections. In recent studies the use of rituximab and azathriprine (combination chemotherapy) has demonstrated a functional and radiographic pulmonary improvement in this group of patients [23,44,45].
In the less common form of the disease, Idiopathic (primary) FB, corticosteroids have been used with anecdotal reports of general improvement in clinical symptoms and resolution of radiographic abnormalities [30]. This is considered as supportive evidence of a hypersensitivity type reaction, or an undiagnosed connective tissue disease with primary respiratory manifestations as the likely aetiology in many cases of idiopathic FB. However, no treatment guidelines have yet been established for the treatment of primary FB, where a relapse of the disease with cessation of therapy and subsequent remission with reinstitution of corticosteroid treatment is commonly seen [33,34]. Macrolide antibiotics have also been used in the treatment of primary FB, with symptomatic improvement possibly related to their anti-inflammatory properties [46].
The prognosis of patients with FB appears to be dependent on two factors, the age at time of presentation and the underlying primary disease. Among all patients with FB middle age and elderly patients diagnosed with primary FB have the most favourable prognosis, while patients under 30 years of age with an underlying immunodeficiency tend to develop a more progressive disease with a higher mortality [47].
Conclusion
Follicular bronchioitis is a benign lymphoproliferative small airway disease and a ubiquitous pathological finding, where the constellation of clinical, radiographic and pathological characteristics is essential for establishing the best course of treatment and predicting disease prognosis. In this article we summarize the stepwise approach in classifying the disease while highlighting its main pathological and radiographic characteristics.
[1]. Whitewall F, A study of the pathology or pathogenesis of bronchiectasisThorax 1952 7:213 [Google Scholar]
[2]. Stephan E, The Child’s Lung; Developmental AnatomyPhysiology and Pathology 1947 6th EdnLondonArnold [Google Scholar]
[3]. Epler GR, Snider GL, Gaensler EA, Cathcart ES, FitzGerald MX, Carrington CB, Bronchiolitis and bronchitis in connective tissue disease: a possible relationship to the use of penicillamineJAMA 1979 242:528 [Google Scholar]
[4]. Troy RD, Bronchus-Associated Lymphoid Tissue (BALT): Structure and FunctionAdvances in Immunology 2010 Vol. 107Elsevier Inc [Google Scholar]
[5]. Bienenstock J, Johnston D, Perey D, Bronchial lymphoid tissue. I. Morphologic characteristicsLab Invest 1973 28:6686 [Google Scholar]
[6]. Randall T, Bronchus-associated lymphoid tissue: structure and functionAdv Immunol 2010 107:187-241. [Google Scholar]
[7]. Randall TD, Carragher DM, Rangel-Moreno J, Development of secondary lymphoid organsAnn Rev Immunol 2008 26:627-50. [Google Scholar]
[8]. Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cellsJ Exp Med 2009 206:2593-601. [Google Scholar]
[9]. Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organsImmunity 2006 25:643-54. [Google Scholar]
[10]. Elliot JG, Jensen CM, Mutavdzic S, Lamb JP, Carroll NG, James AL, Aggregations of lymphoid cells in the airways of nonsmokers, smokers, and subjects with asthmaAm J Respir Crit Care Med 2004 169:712 [Google Scholar]
[11]. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, The nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med 2004 350:2645-53. [Google Scholar]
[12]. Ulrichs T, Kosmiadi G, Trusov V, Jörg S, Pradl L, Titukhina M, Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defense in the lungJ Pathol 2004 204:217-28. [Google Scholar]
[13]. Sarkar PK, Patel N, Furie RA, Talwar A, Pulmonary manifestations of primary Sjögren’s syndromeIndian J Chest Dis Allied Sci 2009 51(2):93-101. [Google Scholar]
[14]. Kobayashi H, Kanoh S, Motoyoshi K, Aida S, Tracheo-broncho-bronchiolar lesions in Sjögren’s syndromeRespirology 2008 13(1):159-61. [Google Scholar]
[15]. Yuksekkaya R, Celikyay F, Yilmaz A, Arslan S, Inanir A, Inonu H, Pulmonary involvement in rheumatoid arthritis: multidetector computed tomography findingsActa Radiol 2013 54(10):1138-49. [Google Scholar]
[16]. Devouassoux G, Cottin V, Lioté H, Marchand E, Frachon I, Schuller A, Characterisation of severe obliterative bronchiolitis in rheumatoid arthritisEur Respir J 2009 33(5):1053-61. [Google Scholar]
[17]. Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosisHistopathology 2004 44(6):585-96. [Google Scholar]
[18]. Garcia D, Young L, Lymphocytic interstitial pneumonia as a manifestation of SLE and secondary Sjogren’s syndromeBMJ Case Rep 2013 2013:pii:bcr2013009598 [Google Scholar]
[19]. Roca B, Ferran G, Simon E, Cortes V, Lymphoid hyperplasia of the lung and Evans syndrome in IgA deficiencyAm J Med 1999 106:121-22. [Google Scholar]
[20]. Máiz L, Muñoz A, Maldonado S, Pacheco A, Lamas A, Fogué L, Bronchiolitis obliterans organizing pneumonia associated with Evans syndromeRespiration 2001 68(6):631-34. [Google Scholar]
[21]. Levinson AI, Hopewell PC, Stites DP, Spitler LE, Fudenberg HH, Coexistent lymphoid interstitial pneumonia, pernicious anemia, and agammaglobulinemiaArch Intern Med 1976 136:213-16. [Google Scholar]
[22]. Travis WD, Fox CH, Devaney KO, Weiss LM, O’Leary TJ, Ognibene FP, Lymphoid pneumonitis in 50 adult patients infected with the human immunodeficiency virus: lymphocytic interstitial pneumonitis versus nonspecific interstitial pneumonitisHum Pathol 1992 23:529-41. [Google Scholar]
[23]. Camarasa Escrig A, Amat Humaran B, Sapia S, León Ramírez JM, Follicular bronchiolitis associated with common variable immunodeficiencyArch Bronconeumol 2013 49(4):166-68. [Google Scholar]
[24]. Park JH, Levinson AI, Granulomatous-lymphocytic interstitial lung disease (GLILD) in common variable immunodeficiency (CVID)Clin Immunol 2010 134(2):97-103. [Google Scholar]
[25]. Travis WD, Pittaluga S, Lipschik GY, Ognibene FP, Suffredini AF, Masur H, Atypical pathologic manifestations of Pneumocystis carinii pneumonia in the acquired immune deficiency syndrome. Review of 123 lung biopsies from 76 patients with emphasis on cysts, vascular invasion, vasculitis, and granulomasAm J Surg Pathol 1990 14:615-25. [Google Scholar]
[26]. Masuda T, Ishikawa Y, Akasaka Y, Ishii T, Tateda K, Ishii Y, Follicular bronchiolitis associated with Legionella pneumophila infectionPediatr Pathol Mol Med 2002 21:41-47. [Google Scholar]
[27]. Koss MN, Hochholzer L, Langloss JM, Wehunt WD, Lazarus AA, Lymphoid interstitial pneumonia: clinicopathological and immunopathological findings in 18 casesPathology 1987 19(2):178-85. [Google Scholar]
[28]. Aerni MR, Vassallo R, Myers JL, Lindell RM, Ryu JH, Follicular bronchiolitis in surgical lung biopsies: clinical implications in 12 patientsRespir Med 2008 102(2):307-12. [Google Scholar]
[29]. Iwata M, Colby TV, Kitaichi M, Diffuse panbrochiolitis: diagnosis and distinction from various pulmonary diseases with centrilobular interstitial foam cell accumulationsHum Pathol 1994 25:357-63. [Google Scholar]
[30]. Ryu JH, Classification and approach to bronchiolar diseasesCurr Opin Pulm Med 2006 12(2):145-51. [Google Scholar]
[31]. Franchi LM, Chin TW, Nussbaum E, Riker J, Robert M, Talbert WM, Familial pulmonary nodular lymphoid hyperplasiaJ Pediatr 1992 121:89-92. [Google Scholar]
[32]. O’Brodovich HM, Moser MM, Lu L, Familial lymphoid interstitial pneumonia: a long-term follow-upPediatrics 1980 65:523-28. [Google Scholar]
[33]. Yousem SA, Colby TV, Carrington CB, Follicular bronchitis/bronchiolitisHum Pathol 1985 16:700-06. [Google Scholar]
[34]. Romero S, Barroso E, Gil J, Aranda I, Alonso S, Garcia-Pachon E, Follicular bronchiolitis: clinical and pathologic findings in six patientsLung 2003 181(6):309-19. [Google Scholar]
[35]. Koss MN, Pulmonary lymphoid disordersSemin Diagn Pathol 1995 12:158-71. [Google Scholar]
[36]. Perez-Padilla R, Gaxiola M, Salas J, Mejia M, Ramos C, Selman M, Bronchiolitis in chronic pigeon breeder’s disease: morphologic evidence of a spectrum of small airway lesions in hypersensitivity pneumonitis induced by avian antigensChest 1996 110:371-77. [Google Scholar]
[37]. Oh YW, Effmann EL, Redding GJ, Godwin JD, Follicular hyperplasia ofbronchus-associated lymphoid tissue causing severe air trappingAm J Roentgenol 1999 172:745-47. [Google Scholar]
[38]. Pipavath SJ, Lynch DA, Cool C, Brown KK, Newell JD, Radiologic andpathologic features of bronchiolitisAJR Am J Roentgenol 2005 185:354-63. [Google Scholar]
[39]. Muller NL, Advances in imagingEur Respir J 2001 18:867-71. [Google Scholar]
[40]. Couture C, Colby TV, Histopathology of bronchiolar disordersSemin Respir Crit Care Med 2003 24:489-98. [Google Scholar]
[41]. Aubry MC, Pulmonary Pathology: LC22-1 Non-neoplastic pulmonary lymphoid proliferationsPathology 2014 46(Suppl 2):S36-37. [Google Scholar]
[42]. Sato A, Hayakawa H, Uchiyama H, Chida K, Cellular distribution of bronchus associated lymphoid tissue in rheumatoid arthritisAm J Respir Crit Care Med 1996 154:1903-07. [Google Scholar]
[43]. Shipe R, Lawrence J, Green J, Enfield K, HIV-associated follicular bronchiolitisAm J Respir Crit Care Med 2013 188(4):510-1. [Google Scholar]
[44]. Costa-Carvalho BT, Wandalsen GF, Pulici G, Aranda CS, Solé D, Pulmonary complications in patients with antibody deficiencyAllergol Immunopathol 2011 39:128-32. [Google Scholar]
[45]. Chase NM, Verbsky JW, Hintermeyer MK, Waukau JK, Tomita-Mitchell A, Casper JT, Use of Combination Chemotherapy for Treatment of Granulomatous and Lymphocytic Interstitial Lung Disease (GLILD) in Patients with Common Variable Immunodeficiency (CVID)J Clin Immunol 2013 33(1):30-39. [Google Scholar]
[46]. Aerni MR, Vassallo R, Successful treatment of follicular bronchiolitis with macrolideAm Coll Chest Phys 2005 :128http://journal.publications.chestnet.org/article.aspx?articleID=1213539 [Google Scholar]
[47]. Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM, Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiencyJ Allergy Clin Immunol 2004 114(2):415-21. [Google Scholar]