Background and Objectives: Alternative therapies are increasingly being explored as extensive use of synthetic chemicals has led to the emergence of substantial side effects. Phytomedicine has been well practiced as traditional medicine in various cultures for treatment of oral diseases. It has gained importance of late as an alternative to the conventional therapy. Various plant and fruit extracts have been monitored recently to assess their potential against the common oral pathogens. Aim of this study was to assess in-vitro efficacy of pomegranate peel, lotus leaf, guava leaf and coffee extracts on oral microorganisms.
Materials and Methods: Concentrations of 1%, 5%, 10%, 15% and 20% were prepared for each, followed by efficacy testing using disc diffusion method against Streptococcus mutans, Streptococcus mitis, Porphyromonas gingivalis, Prevotella intermedia and Candida albicans.
Results: All the four extracts were found to be effective against S.mutans and S.mitis, with maximum efficacy against S.mutans and S.mitis displayed by pomegranate and lotus. Antifungal efficacy was demonstrated by coffee and pomegranate. Guava, lotus and coffee were effective against P.intermedia, while only coffee was found to be effective against P. gingivalis. All the results were found to be statistically significant (p < 0.05).
Interpretation & Conclusion: Pomegranate, guava, lotus and coffee displayed significant anticariogenic effect while coffee was found to be most effective against periodontal pathogens as well as Candida albicans. Results revealed that natural products may be used as economical and suitable adjuvant to synthetic medicines and compounds and their judicious use might not only help to inhibit the side effects of synthetic chemicals but also prove to be cost effective in developing economies.
Introduction
Widespread use of synthetic chemicals and drugs has resulted in emergence of side effects, uncommon infections and antimicrobial resistance. Phytoplants appear to be suitable alternatives as they are available and accessible at affordable prices [1].Punica granatum (pomegranate) has been quoted as an effective herbal extract in Iranian medicine. Recent studies have revealed the bactericidal, antifungal, antiviral, immune modulation and anthelminthic properties of pomegranate [1]. Lotus leaves have been conventionally used for the treatment of mouth inflammation, tumescence and halitosis as per the traditional Chinese medicine [2].
Folklore practices support the use of extracts of Piper betle to maintain the oral hygiene. In vitro studies have proved their antibacterial effect on plaque bacteria [3]. Coffee has demonstrated significant antibacterial properties against cariogenic bacteria. Among all the natural products with antibacterial properties, coffee is the most popular owing to its safety and pleasant odour and taste. Coffea arabica and Coffea canephora are the two most commercialized coffee species and a recent study indicated higher efficacy of the latter [4,5].
The association between oral infections and microbial activities has been well-established [6]. The levels of P.gingivalis, P.intermedia and other anaerobic bacteria are seen to increase in adult onset periodontitis. While P.gingivalis is strongly associated with localized aggressive periodontitis (LAP), P.intermedia is predominantly associated with the development of necrotizing ulcerative gingivitis (NUG) [7]. The association between Streptococcus species and dental caries has been well documented with S.mutans playing a vital role in caries formation and progression [8].Several extracts have been evaluated for their antibacterial activity against the Streptococcus species [3,9,10].Studies have also shown that C. albicans has a similar capacity as S.mutans to colonize hydroxyapatite and its possible relation with periodontal diseases [8].
Polyphenols and tannins extracted from different natural products help in prevention of oral diseases, particularly biofilm-related oral diseases [4,11].Potential of tannin rich pomegranate against the cariogenic bacteria and candida has been reported in the literature [1,8,11,12].Similarly, the potential of lotus on periodontal pathogens and, the anticariogenic potential of coffee and guava have been demonstrated [2-5,13].However, the impact of these extracts on the usual cariogenic, periodontal and fungal bacteria have not been reported as a whole. The present study is the first to comprehensively report the antimicrobial potential of the afore mentioned products on the common oral pathogens. Hence, it was conceptualized to assess the in-vitro efficacy of pomegranate peel, Lotus leaf, Guava leaf and Coffee extract on the popular oral microorganisms S.mutans, S.mitis, P.gingivalis, P. intermedia and C.albicans.
Material and Methods
The present study was conducted in the Department of Public Health Dentistry in Manipal College of Dental Sciences, Mangalore, India after obtaining the ethical approval from the Institutional Ethics Committee.
Collection of Materials
Punica granatum (pomegranate) fruits were obtained from local market in Mangalore city in the month of November. After washing, mesocarp of pomegranate were separated and dried. Leaves of Nelumbo nucifera (lotus) and Psidium guajava (guava) were obtained from local cultivations in Mangalore district. They were washed and dried before further processing. Coffee beans (Coffea canephora) were obtained from local cultivations in Chikkamagaluru district in Karnataka. Coffee beans were dried and all the specimens were powdered separately in an electric grinder. Specimens were identified by a botanist and a pharmacognosist for their authenticity.
Pure cultures of S. mutans, S. mitis, P. gingivalis, P. intermedia and C. albicans were obtained from the Department of Microbiology, Maratha Mandal’s NGH Institute of Dental Sciences & Research Centre, Belgaum, Karnataka, India.
Preparation of Extracts
Aqueous extracts of all the four materials were prepared in the Department of Pharmacology, Kasturba Medical College, Mangalore, Karnataka, India. The procedure of aqueous decoction followed to prepare the extracts of pomegranate, guava and lotus was based on a previous study by Pai et al., [11]. Decoctions were prepared by boiling 10 grams of the material in 100 ml sterile distilled water over a low flame for 15min. The flasks were then plugged, removed from heat and allowed to cool for 45min. After cooling, the contents of the flasks were filtered with double filter paper and sterile filters to remove any impurities [11,14]. Each extract was then diluted with sterile water to obtain the five different concentrations of 1%, 5%, 10%, 15% and 20%.
Aqueous extracts of Coffea canephora were obtained by a coffee brewing procedure based on a previous study by Antonio et al., [4]. Preparation of 20% extract was done by percolating 100ml of pre-boiling (95ºC) sterile water through 20g of ground non-roasted coffee. Whatman #1 qualitative filter paper was used to filter the extracts. After preparation of 20% aqueous extract of Coffea canephora, further dilution was done using sterile water to obtain the concentrations of 1%, 5%, 10% and 15%.
All the extracts were stored separately in sterile containers and labeled accordingly. These containers were stored in refrigerators and transported to the Department of Microbiology, Maratha Mandal’s NGH Institute of Dental Sciences & Research Centre, Belgaum for microbiological assays.Chlorhexidine (0.2%) was used as positive control and the standard values of respective antimicrobial agents (gold standard) in disc diffusion method were used for comparison. All the samples were stored in a refrigerator at 4ºC until the analyses were accomplished.
Antimicrobial Tests
In-vitro determination of antimicrobial activity was done using disc diffusion method. Blood agar was used to culture both the organisms.Different types of media were used for culturing of the different micro-organisms in this study.
For S.mutans and S.mitis - Brain Heart Infusion agar
For P.gingivalis and P.intermedia - Blood agar
For C.albicans - Sabouraud’s dextrose agar (SDA)
The overall procedure of inoculum preparation and inoculation of culture media remained the same. Swab method was used to transfer the colonies on to the agar plates. Turbidity was adjusted visually with the broth to equal that of a 0.5 McFarland turbidity standard that had been vortexed. Within 15min, sterile cotton swab was dipped into the inoculum and rotated against the tube wall above the liquid to remove the excess inoculum. Swabbing of the entire surface of agar plate was done thrice in order to ensure uniform distribution. Inoculated plates were allowed to stand for about 3min before making wells. Hollow tube of 5mm diameter was heated and pressed above the inoculated agar plates and was removed immediately by making a well in the plate. Addition of 30μl of compound was done into each well and within 15min, plates were incubated at 37ºC in an incubator for 24h. For anaerobic bacteria, plates were incubated in the anaerobic jar and the jar was kept in the incubator for 48h [15].
After the incubation period, plates were read only if the lawn of growth was confluent or nearly confluent. The diameter of inhibition zone was measured to the nearest whole millimetre by holding the measuring device. The experiment was repeated four times for every concentration of each extract on all the four bacteria and the means were obtained. These mean values of every extract were compared with the values of their respective gold standard (ciprofloxacin/fluconazole) and with the positive control (chlorhexidine 0.2%). All the measurements of zone of inhibition were performed by a single examiner only. Kappa statistics was used to assess the intra-examiner agreement scores. Examiner calibration was done prior to the study and also during the study by re-examination of 5% of the samples.
Statistical Analysis
Statistical analysis was done using the software SPSS (version 11.5). The disc diffusion values of all the different concentrations were entered in the SPSS software for statistical analysis. Descriptive statistics were retrieved and data was analysed using one way analysis of variance (ANOVA) and Tukey post-hoc test [16]. Statistical significance level was established at p < 0.05.
Results
The descriptive results of antimicrobial efficacy of various concentrations of extracts of Punica granatum, Psidium guajava, Nelumbo nucifera and Coffea canephora have been tabulated in [Table/Fig-1].
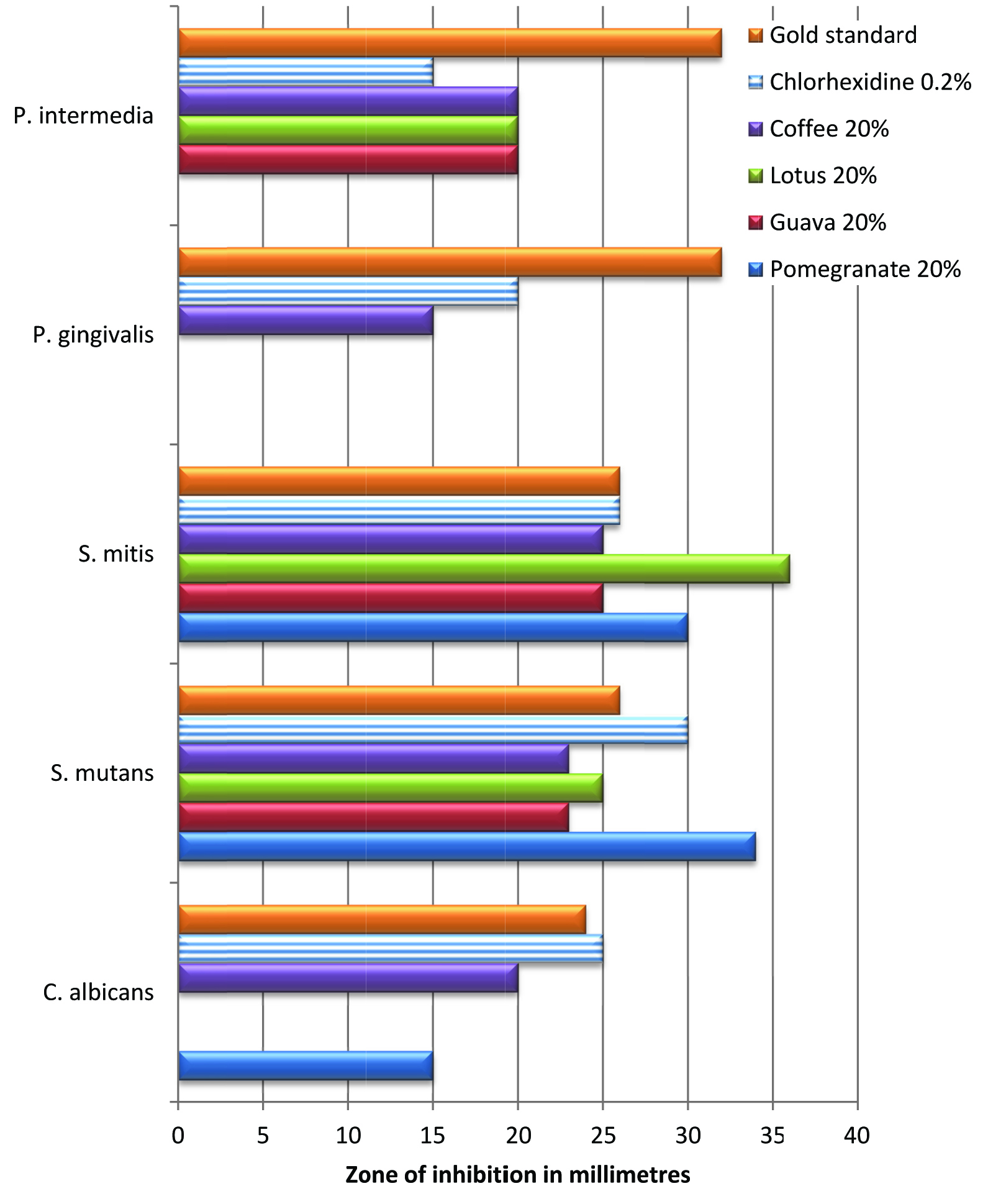
Nearly all extracts showed high efficacy against the cariogenic bacteria S.mutans and S.mitis (p=0.00). Only some concentrations of Psidium guajava, Nelumbo nucifera and Coffea canephora showed efficacy against the periodontal bacteria (p=0.00). While all concentrations of Punica granatum showed consistent antifungal efficacy, only higher concentrations of Coffea canephora were found to have antifungal properties (p = 0.00). Overall, it was observed that the antibacterial efficacy of all the extracts increased with an increase in the concentration. Comparison of mean efficacy values of 20% extracts with chlorhexidine 0.2% and the respective gold standards (ciprofloxacin/fluconazole) has been shown in [Table/Fig-2].
For the cariogenic bacteria, values obtained for all the extracts were comparable with chlorhexidine 0.2% and the respective gold standards. Albeit Punica and Coffea showed some antifungal efficacy, their values were not comparable to chlorhexidine 0.2% or gold standard. Except Punica, the other three extracts showed higher efficacy against P.intermedia as compared to chlorhexidine 0.2%. However, only Coffea showed some efficacy against P.gingivalis.
Zones of inhibition of 0.2% chlorhexidine, gold standard (fluconazole/ciprofloxacin) and the extracts of Punica granatum, Psidium guajava, Nelumbo nucifera and Coffea canephora in millimetres (Means and standard deviation) * R, resistant; † NA, not applicable
| Bacteria →
Concentrations↓ | C. albicans | S. mutans | S. mitis | P. gingivalis | P.intermedia |
| Pomegranate 1% | 15 (1.15) | 20 (1.41) | 25 (0.82) | R | R |
| Pomegranate 5% | 15 (0.82) | 32 (1.26) | 25 (0.00) | R | R |
| Pomegranate 10% | 15 (0.00) | 33 (0.82) | 28 (2.16) | R | R |
| Pomegranate 15% | 15 (0.00) | 33 (0.82) | 30 (0.96) | R | R |
| Pomegranate 20% | 15 (0.00) | 34 (0.82) | 30 (1.63) | R | R |
| Guava 1% | R | R | R | R | R |
| Guava 5% | R | 15 (0.00) | 15 (0.00) | R | R |
| Guava 10% | R | 20 (0.82) | 20 (0.82) | R | R |
| Guava 15% | R | 22 (1.63) | 23 (1.42) | R | R |
| Guava 20% | R | 23 (1.42) | 25 (0.00) | R | 20 (1.63) |
| Lotus 1% | R | 10 (0.00) | 30 (1.82) | R | R |
| Lotus 5% | R | 15 (1.82) | 35 (2.16) | R | R |
| Lotus 10% | R | 20 (1.41) | 35 (2.45) | R | R |
| Lotus 15% | R | 23 (2.00) | 35 (1.41) | R | R |
| Lotus 20% | R | 25 (1.41) | 36 (2.00) | R | 20 (1.82) |
| Coffee 1% | R | 15 (0.00) | R | 8 (1.41) | R |
| Coffee 5% | R | 20 (2.30) | 15 (0.00) | 8 (1.15) | R |
| Coffee 10% | R | 23 (1.82) | 18 (0.82) | 10 (0.82) | R |
| Coffee 15% | 15 (0.00) | 23 (1.41) | 23 (2.16) | 13 (0.82) | 18 (1.41) |
| Coffee 20% | 20 (0.00) | 23 (2.16) | 25 (0.00) | 15 (1.15) | 20 (2.45) |
| Fluconazole | 24 | NA | NA | NA | NA |
| Ciprofloxacin | NA | 26 | 26 | 32 | 32 |
| Chlorhexidine | 25 | 30 | 26 | 20 | 15 |
Mean efficacy values of 20% extracts, respective gold standard and positive control [Chlorhexidine 0.2%] on different bacteria
Discussion
Synthetic antimicrobial agents have resulted in considerable side effects, antimicrobial resistance and the emergence of previously uncommon infections owing to their improper usage [2]. Instead, plant extracts may prove to be better and safer alternatives if they are supported by scientific-based evidence. Several common natural products like lemon peel, neem stick and tulsi have been tested for their antibacterial properties [17-19]. Most of these plants that are used for traditional medicines can be cultivated locally and their extracts can be obtained locally by public for therapeutic use. Also, resistance by pathogenic bacteria to currently used antibiotics and chemotherapeutics has increased the global requirement for alternative safe, efficacious and cost-effective treatment options for such infections, particularly in developing countries [6].
This study found Punicagranetumto be efficacious against Candida and the cariogenic bacteria, which substantiate the previous findings [1,8,11,13].High reserves of tannins and polyphenols in Punica have been acknowledged for their antibacterial activity [1]. While tannins can precipitate proteins due to their ability to act on the cell surface and across the cell membranes, polyphenols are known to interact with proteins and disturb microbial co-aggregation [8,12,20].However, the precise mechanism of action against C.albicans is not fully understood [1,11].
In vitro studies have shown the potential of Psidiumguajava in disrupting the bonding and adhesion of early settlers in dental plaque [3,21].Its capacity to act against the chief cariogenic pathogen S.mutans and the common periodontal bacteria P.intermedia was demonstrated in this study. Moreover, it showed no potential against Candida and P.gingivalis. It has been offered that Psidiumguajava extracts have the competence to modify the surface characteristics of the bacteria by altering the complementary binding sites in the dental pellicle [3,16].
Lotus leaf extracts illustrated efficacy against the cariogenic bacteria S.mutans and S.mitis and the periodontal pathogen P.intermedia. However, the aqueous concentrations used in this study did not show any potential against P.gingivalis, which is conflicting to the findings of a previous study in which higher concentrations of alcoholic lotus extract were used [2]. It is proposed that flavonoids like quercetin or quercetin 3-O-glycosides might be responsible for the antibacterial activity demonstrated by lotus leaf extracts [2].
Roasted coffee extracts have proved to be effective against cariogenic bacteria in several studies, while non-roasted coffee extracts have been claimed to have weak antibacterial activity. Several studies have demonstrated potential of coffee against various bacteria [4,5,22,23]. However, its potential against periodontal pathogens is hitherto, a mystery. The current study found non-roasted coffee extracts effective against all the five bacteria. Polyphenols are the chief constituents in coffee responsible for the antibacterial activity [12]. As the concentration of coffee increased, extracts showed higher efficacy and at 15 and 20%, antimicrobial activity was comparable to chlorhexidine 0.2% although it was about half as compared to the gold standard.
For all the extracts used, it remains a matter of discussion whether a rise in concentration will prove to be better than chlorhexidine 0.2% and/or gold standard or the ‘ceiling effect’ will come in to play. Hence, it is difficult to conclude without investigating the efficacy of higher concentrations. Assuming that an increase in concentration improves efficacy, most of the problems encountered with the use of synthetic drugs and chemicals might tend to taper. It is noteworthy that sweetened coffee extracts lead to a surge in the oral microbial counts due to delivery of sucrose [4]. Hence, when taken judiciously, coffee may help to reduce the oral microbial load.
Thus, maintenance or improvement of oral health by use of natural products that also form a part of habitual diet seems to be a suitable way to preserve oral health. Patients will tend to be compliant as they are not burdened with added cost or time. However, this cannot be confirmed without appropriate studies. Additionally, proper utilization of available natural resources to reduce the ever increasing prevalence of oral diseases in rural parts of developing countries can help to diminish the dangers of a poor dentistpopulation ratio [24].
Active ingredients from such potent natural resources should also be evaluated for their anti-inflammatory and immune-modulatory properties. It is recommended that the readily accessible natural products may be integrated with presently available synthetic materials that are used to maintain the oral hygiene. However, any natural product should be assessed for its safety and clinical application should not be done without sufficient scientific evidence. The mechanism of action of a particular natural product needs to be studied at a molecular level in order to assure the accuracy of application and consequently, the outcomes. Although none of the products used in this study are known to have side effects, oral retention and duration of action of these phytoplants is an important factor that needs further exploration.
Conclusion
Pomegranate, guava, lotus and coffee displayed significant anticariogenic effect while coffee was found to be most effective against periodontal pathogens as well as Candida albicans. Overall, results revealed the probable use of natural products as economical and suitable adjuvant to synthetic medicines and compounds. Thus, judicious dealing of such natural products might not only help to inhibit the side effects of synthetic chemicals but also prove to be cost effective, especially in developing economies.
[1]. S Abdollahzadeh, RY Mashouf, H Mortazavi, MH Moghaddam, N Roozbahani, M Vahedi, Antibacterial and Antifungal Activities of Punica granatum Peel Extracts Against Oral PathogensJ Dent (Tehran). 2011 8(1):1-6. [Google Scholar]
[2]. M Li, Z Xu, Quercetin in a Lotus Leaves Extract May be Responsible for Antibacterial ActivityArch Pharm Res 2008 31(5):640-44. [Google Scholar]
[3]. FA Razak, ZH Rahim, The anti-adherence effect of Piper betle and Psidiumguajava extracts on the adhesion of early settlers in dental plaque to saliva coated glass surfaces.J Oral Sci 2003 45(4):201-06. [Google Scholar]
[4]. AG Antonio, NL Iorio, A Farah, KR Netto dos Santos, LC Maia, Effect of Coffea canephora Aqueous Extract On Microbial Counts in Ex Vivo Oral Biofilms: A Case Study.Planta Med. 2012 78:755-60. [Google Scholar]
[5]. AG Antonio, RS Moraes, D Perrone, LC Maia, KRN Santos, NLP Iorio, Species, roasting degree and decaffeination influence the antibacterial activity of coffee against Streptococcus mutansFood Chemistry 2010 118:782-88. [Google Scholar]
[6]. K Borhan-Mojabi, M Sharifi, T Karagah, Efficacy of different concentrations of garlic extract in reduction of oral salivary microorganismsArch Iran Med 2012 15(2):99-101. [Google Scholar]
[7]. FA Carranza, In Carranza’s clinical periodontology 2002 17th EditionPhiladelphia, USAW.B. Saunders company [Google Scholar]
[8]. LC Vasconcelos, FC Sampaio, MC Sampaio, S Pereira Mdo, JS Higino, MH Peixoto, Minimum Inhibitory Concentration of Adherence of Punica granatum Linn (pomegranate) Gel Against S. mutans, S. mitis and C. albicansBraz Dent J. 2006 17(3):223-27. [Google Scholar]
[9]. LE Wolinsky, S Mania, S Nachnani, S Ling, The inhibiting effect of aqueous Azadirachtaindica (Neem) extract upon bacterial properties influencing in vitro plaque formation.J Dent Res 1996 75(2):816-22. [Google Scholar]
[10]. B Houshmand, F Mahjour, O Dianat, Antibacterial effect of different concentrations of garlic (Allium sativum) extract on dental plaque bacteriaIndian J Dent Res. 2013 24(1):71-5. [Google Scholar]
[11]. MB Pai, GM Prashant, KS Murlikrishna, KM Shivakumar, GN Chandu, Antifungal efficacy of Punica granatum, Acacia nilotica, Cuminum cyminum and Foeniculum vulgare on Candida albicans: An in vitro studyIndian J Dent Res 2010 21(3):334-36. [Google Scholar]
[12]. LC Vasconcelos, MC Sampaio, FC Sampaio, JS Higino, Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis.Mycoses. 2003 46(5-6):192-96. [Google Scholar]
[13]. MY Li, ZT Xu, The inhibition of dentifrice containing the lotus leaf-derived inhibitor on periodontitis-related bacteria in vitroInt Dent J. 2007 57(5):303-06. [Google Scholar]
[14]. DK Runyoro, M Matee, OD Ngassapa, CC Joseph, CH Mbwambo, BMC complementary and alternative medicine screening of Tanzanian medicinal plants for anti-candida activity.BMC Complement Altern Med 2006 6(11) [Google Scholar]
[15]. HD Isenberg, Clinical microbiology procedures handbook, Volume 1.American society for microbiology/ Washington, D.C 1992 [Google Scholar]
[16]. TDV Swinscow, MJ Campbell, In Statistics at Square OneBMJ Books 2002 10th Edition [Google Scholar]
[17]. Y Miyake, M Hiramitsu, Isolation and extraction of antimicrobial substances against oral bacteria from lemon peelJ Food Sci Technol 2011 48(5):635-39. [Google Scholar]
[18]. LE Wolinsky, S Mania, S Nachnani, S Ling, The inhibiting effect of aqueous Azadirachtaindica (Neem) extract upon bacterial properties influencing in vitro plaque formation.J Dent Res 1996 75(2):816-22. [Google Scholar]
[19]. P Agarwal, L Nagesh, Comparative evaluation of efficacy of 0.2% Chlorhexidine, Listerine and Tulsi extract mouth rinses on salivary Streptococcus mutans count of high school children-RCTContemp Clin Trials 2011 32(6):802-08. [Google Scholar]
[20]. S Naz, R Siddiqi, S Ahmad, SA Rasool, SA Sayeed, Antibacterial activity directed isolation of compounds from Punica granatumJ Food Sci. 2007 72(9):M341-45. [Google Scholar]
[21]. FA Razak, RY Othman, ZH Rahim, The effect of Piper betle and Psidiumguajava extracts on the cell surface hydrophobicity of selected early settlers of dental plaqueJ Oral Sci 2006 48(2):71-75. [Google Scholar]
[22]. AAP Almeida, CC Naghetini, VR Santos, AG Antonio, A Farah, Glória MBA. Influence of natural coffee compounds, coffee extracts and increased levels of caffeine on the inhibition of Streptococcus mutansFood Research International 2012 49:459-61. [Google Scholar]
[23]. GF Ferrazzano, I Amato, A Ingenito, A De Natale, A Pollio, Anti-cariogenic effects of polyphenols from plant stimulant beveragesFitoterapia. 2009 80:255-62. [Google Scholar]
[24]. NK Ahuja, R Parmar, Demographics & Current Scenario with Respect to Dentists, Dental Institutions & Dental Practices in IndiaIndian Journal of Dental Sciences 2011 3(2):8-11. [Google Scholar]